Research Articles
Comparative Life Cycle Assessment of Biosynthesis Methods: A Roadmap for Sustainable Pharmaceutical and Bio-Based Chemical Production
This article provides a comprehensive framework for conducting comparative Life Cycle Assessments (LCAs) of biosynthesis methods, tailored for researchers and drug development professionals.
Benchmarking Novel Biosynthetic Pathways: From AI-Driven Discovery to Industrial Validation
This article provides a comprehensive framework for evaluating novel biosynthetic pathways against established routes, a critical task for researchers and drug development professionals scaling natural product synthesis.
Analytical Techniques for Biosynthetic Product Validation: From Discovery to Clinical Application
This comprehensive review addresses the critical role of analytical techniques in validating natural product biosynthesis for researchers, scientists, and drug development professionals.
Comparative Substrate Specificity of Enzyme Homologs: Mechanisms, Methods, and Impact on Drug Discovery
This article provides a comprehensive analysis of the comparative substrate specificity of enzyme homologs, a critical factor in enzymology and pharmaceutical development.
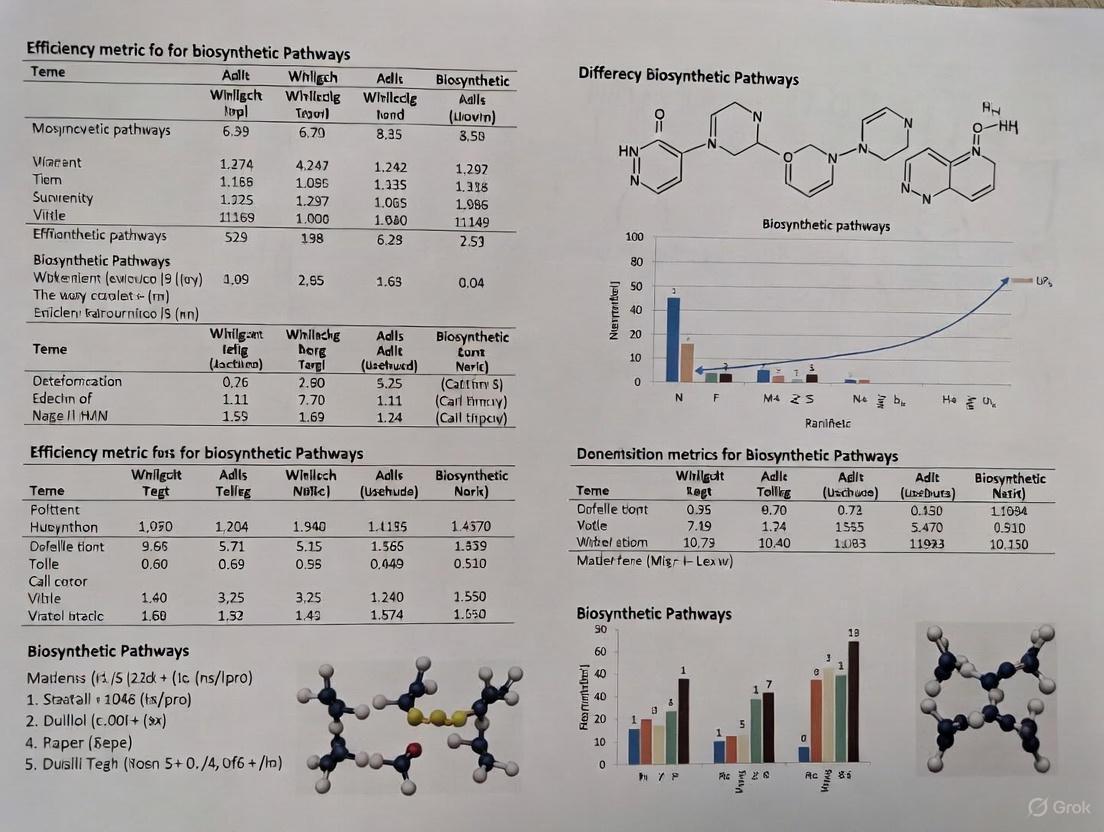
Measuring Success: Key Efficiency Metrics and Optimization Strategies for Biosynthetic Pathways
This comprehensive review addresses the critical challenge of quantifying and enhancing efficiency in biosynthetic pathways for researchers, scientists, and drug development professionals.
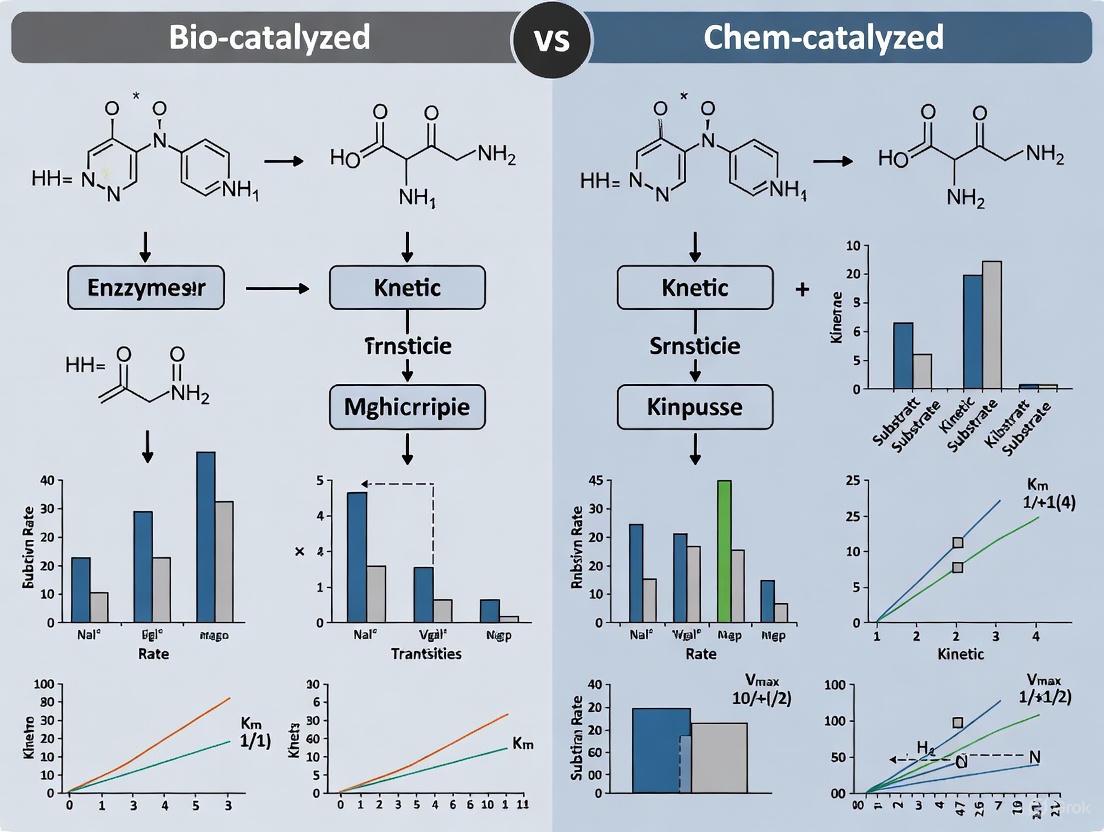
Comparative Kinetics in Drug Development: Analyzing Bio-catalyzed vs. Chem-catalyzed Reaction Pathways
This article provides a comprehensive comparative analysis of the kinetics of bio-catalyzed and chem-catalyzed reactions, tailored for researchers, scientists, and drug development professionals.
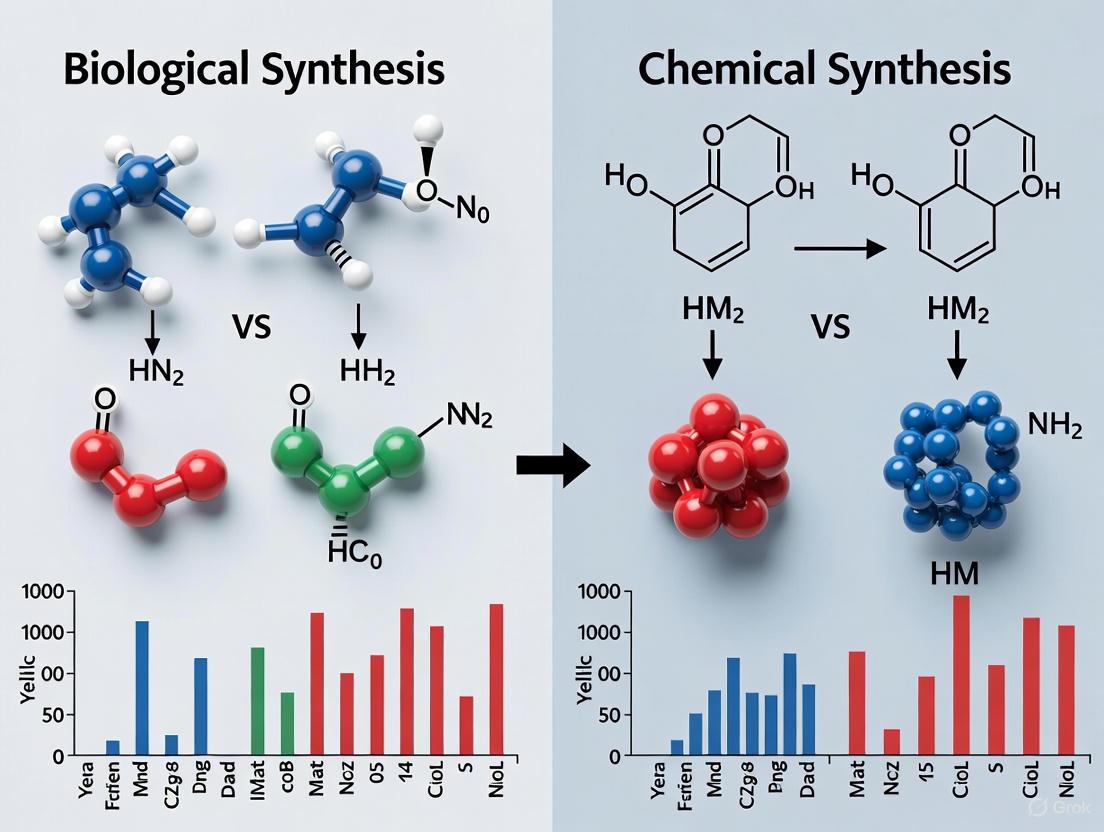
Biological vs. Chemical Synthesis: A Comparative Analysis for Modern Drug Development
This article provides a comprehensive comparative analysis of biological and chemical synthesis, two pivotal methodologies in pharmaceutical development.
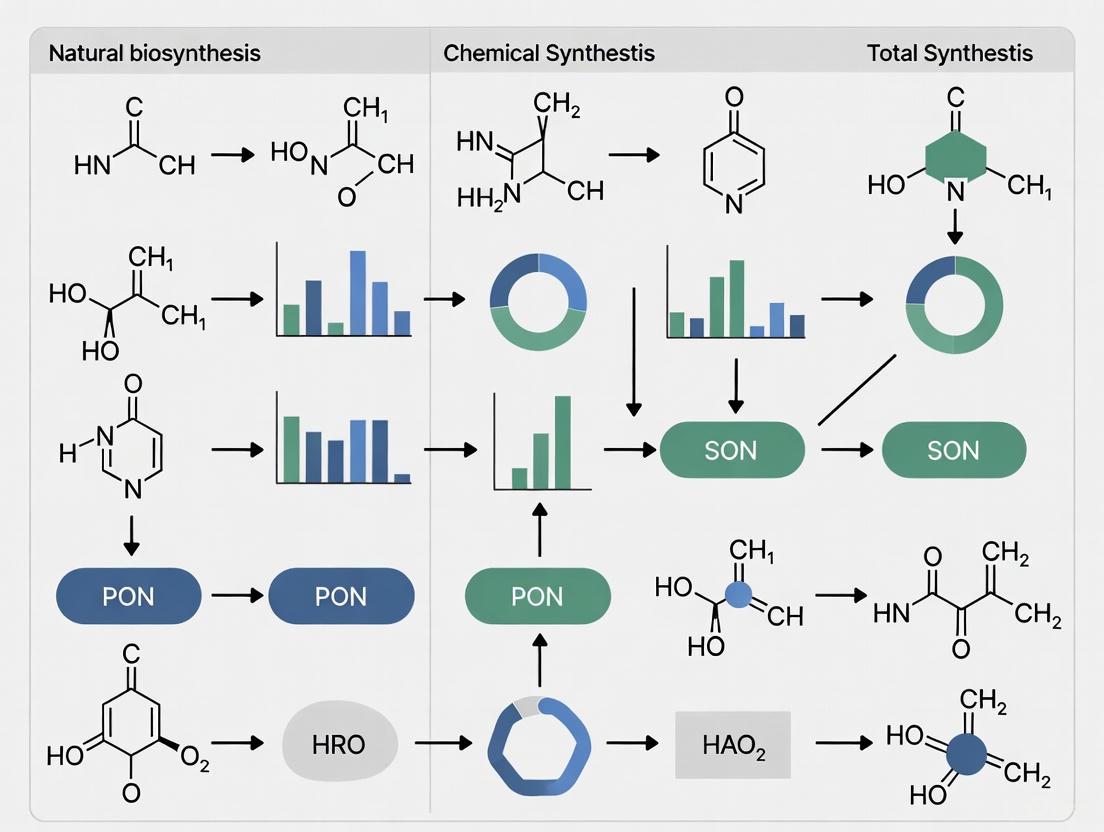
Divergent Pathways: Unlocking Nature's Biosynthetic Strategies for Modern Drug Synthesis
This article explores the parallel and increasingly convergent strategies employed by nature and synthetic chemists in the total synthesis of complex molecules.
Biosynthetic Process Scale-Up: Strategies for Translating Lab Success to Industrial Production
This article provides a comprehensive guide for researchers and drug development professionals on scaling up biosynthetic processes.
Dynamic Control of Biosynthetic Reactor Parameters: Enhancing Robustness, Yield, and Scalability in Bioprocessing
This article provides a comprehensive overview of dynamic control strategies for optimizing biosynthetic reactor parameters, tailored for researchers, scientists, and drug development professionals.