Biosynthetic Pathway Elucidation and Discovery: From Foundational Concepts to AI-Driven Engineering
This article provides a comprehensive overview of the strategies and technologies driving the elucidation of biosynthetic pathways for plant natural products and other valuable compounds.
Biosynthetic Pathway Elucidation and Discovery: From Foundational Concepts to AI-Driven Engineering
Abstract
This article provides a comprehensive overview of the strategies and technologies driving the elucidation of biosynthetic pathways for plant natural products and other valuable compounds. Tailored for researchers, scientists, and drug development professionals, it covers foundational principles, cutting-edge multi-omics and AI methodologies, optimization challenges in heterologous production, and rigorous validation techniques. By synthesizing recent advances, this review serves as a guide for unlocking nature's chemical diversity to enable the sustainable bioproduction of pharmaceuticals, agrochemicals, and other high-value substances.
The Foundation of Chemical Diversity: Uncovering Nature's Biosynthetic Blueprints
Defining Biosynthetic Pathways and Their Significance in Drug Discovery
Biosynthetic pathways are the sequential enzymatic reactions used by living organisms to build complex natural products (NPs) from simple, readily available precursors [1] [2]. These pathways are central to synthetic biology, which aims to produce value-added compounds for various applications, including pharmacology [1]. The astounding chemodiversity of NPs stems from a relatively small number of core biosynthetic pathways, such as those for acetic acid/malonic acid (AA/MA), mevalonic acid/methylerythritol phosphate (MVA/MEP), shikimic acid (CA/SA), and amino acids (AAs), which generate polyketides, terpenoids, phenylpropanoids, and alkaloids, respectively [3]. Unfortunately, complete biosynthetic pathways, including all intermediates, are not established for most of the hundreds of thousands of known NPs [3]. This knowledge gap presents a significant obstacle, particularly in drug discovery, where over 60% of FDA-approved small molecule drugs are NPs or their derivatives [3]. Elucidating these pathways is therefore not merely an academic exercise but a critical endeavor for developing sustainable supplies of vital therapeutics.
Computational and AI-Driven Elucidation Methods
The challenge of defining unknown biosynthetic pathways has been met with advanced computational methods. Traditionally, rule-based models matched query molecules to generalized reaction rules, but these were limited to existing knowledge bases [3]. Recently, deep learning has emerged as a transformative, rule-free approach. For instance, the tool BioNavi-NP uses transformer neural networks trained on biochemical and organic reactions to predict biosynthetic precursors in an end-to-end fashion [3]. Its performance significantly outperforms previous rule-based models, as shown in Table 1 [3].
Table 1: Performance comparison of single-step bio-retrosynthesis prediction models.
| Model | Training Data | Top-1 Accuracy (%) | Top-10 Accuracy (%) |
|---|---|---|---|
| Transformer | BioChem (31,710 reactions) | 10.6 | 27.8 |
| Transformer | BioChem (without chirality) | - | 16.3 |
| Transformer | BioChem + USPTO_NPL | 17.2 | 48.2 |
| Ensemble Transformer | BioChem + USPTO_NPL | 21.7 | 60.6 |
| RetropathRL (Rule-based) | - | - | ~42.1 |
Based on a reliable single-step model, multi-step pathway planning can be performed using search algorithms like AND-OR trees. BioNavi-NP successfully identified pathways for 90.2% of 368 test compounds and recovered reported building blocks with 72.8% accuracy [3]. These AI-driven tools are navigable and user-friendly, freely available to the scientific community to facilitate pathway elucidation [3]. Furthermore, genome mining serves as a powerful, data-driven strategy to uncover cryptic biosynthetic gene clusters (BGCs) and enzymes with novel or stereodivergent activities, expanding the enzymatic toolbox for constructing complex chiral architectures relevant to pharmaceuticals [4].
Experimental Workflows for Pathway Discovery
Computational predictions require experimental validation and de novo discovery, which rely on robust genomic and transcriptomic workflows. A prime example is the elucidation of the paclitaxel (Taxol) biosynthesis pathway, a clinically important anticancer drug [5].
Protocol: Multi-Omics Pathway Elucidation
The following protocol, exemplified by McClune et al. (2025), details the steps for elucidating a complex plant biosynthetic pathway [5].
Sample Perturbation and Pooling:
- Objective: Generate a wide array of gene expression states for the target pathway.
- Procedure: Apply multiple chemical, environmental, or hormonal treatments to the source organism (e.g., Taxus needles). Vary factors such as treatment type, incubation time, and tissue age. In the paclitaxel study, 272 samples were generated using 17 different treatments, 4 incubation times, and 2 needle age classes. These samples are then pooled for a single, consolidated analysis.
Single-Nucleus RNA Sequencing (sn-RNAseq):
- Objective: Obtain high-resolution transcriptomic data at the level of individual cell nuclei, rather than bulk tissue.
- Procedure: Isolate nuclei from the pooled sample and perform sn-RNAseq. This generates transcriptome data for thousands of individual nuclei, representing distinct cell states.
Co-Expression Network Analysis:
- Objective: Identify groups of genes (modules) that are expressed together and are enriched for the target pathway.
- Procedure: Use computational methods like consensus nonnegative matrix factorization (NMF) on the sn-RNAseq data to define co-expression modules. Search for modules that are enriched with previously characterized genes from the pathway of interest.
Candidate Gene Selection and Validation:
- Objective: Identify and functionally characterize novel genes within the co-expression modules.
- Procedure:
- Browse the enriched modules for co-expressed genes with annotations (e.g., cytochrome P450s, acyltransferases) relevant to the missing biochemical steps.
- Clone the candidate genes and express them in a heterologous system (e.g., Nicotiana benthamiana).
- Use metabolomic analyses (e.g., LC-MS) to detect the production of expected pathway intermediates or final products, confirming gene function.
This "multiplexed perturbation × single nucleus" (mpxsn) approach allows researchers to bypass the traditionally challenging step of pre-identifying ideal pathway-induction conditions and efficiently narrows thousands of candidate genes down to a manageable number for testing [5].
Protocol: High-Quality Genome Assembly for Mining
For effective genome mining, a high-quality, contiguous genome assembly is a prerequisite. The workflow for the Ophiorrhiza pumila (a camptothecin-producing plant) genome serves as a benchmark [2].
Sequencing and Initial Assembly:
- Perform sequencing using a combination of 2nd generation short-read (Illumina) and 3rd generation long-read (PacBio) technologies.
- Assemble the long reads de novo using a tool like Canu.
Multi-Stage Scaffolding:
- Scaffold the initial contigs using Bionano optical maps to create a hybrid assembly.
- Further scaffold the assembly using Hi-C proximity ligation data to achieve chromosome-level continuity.
Polishing and Error Correction:
- Polish the assembled sequence using the original long reads.
- Perform final error correction using high-accuracy short reads and a tool like Pilon.
Experimental Validation:
- Use fluorescence in situ hybridization (FISH) with probes designed from the assembled sequence to experimentally validate contig orientation and correct misassemblies, providing a critical quality control check.
This multi-stage approach resulted in a chromosome-level assembly with a contig N50 of 18.49 Mb, a significant improvement over previously published genomes for medicinal plants, enabling sophisticated comparative genomics and BGC analysis [2].
Case Studies in Drug Discovery
Paclitaxel (Taxol) Biosynthesis
Paclitaxel is a cornerstone anticancer drug whose complex tetracyclic core skeleton and various functional groups made its biosynthetic pathway particularly challenging to elucidate [5]. A recent breakthrough using the mpxsn workflow on Taxus media needles identified eight new genes and refined the order of several biosynthetic steps [5]. Key discoveries included specific hydroxylases, an oxidase, an acyl-CoA ligase, and a non-enzymatic nuclear transport factor 2-like protein (FoTO1) that acts as a scaffolding protein to facilitate early oxidation steps [5]. This protein boosted intermediate production by up to 17-fold in a heterologous system. The successful expression of these genes in Nicotiana benthamiana led to unprecedented production levels of baccatin III, a key paclitaxel precursor, demonstrating the potential for sustainable, cost-effective biomanufacturing of this critical drug [5].
Camptothecin Biosynthesis
Camptothecin is a potent monoterpene indole alkaloid (MIA) used against various cancers. Its biosynthetic origin has been debated, proposed to derive from strictosidine in Ophiorrhiza pumila or strictosidinic acid in Camptotheca acuminata [2]. The construction of a chromosome-level genome assembly for O. pumila enabled the use of integrative omics, phylogenetics, and BGC evaluation to puzzle out the evolutionary origins of MIA metabolism [2]. This high-quality genome allowed the identification of 33 MIA biosynthetic gene clusters and revealed a short-list of high-confidence genes for functional validation. Such work is fundamental for reconstructing the pathway in a heterologous host, which is a promising alternative to inefficient extraction from low-yielding plants [2].
Table 2: Key Reagent Solutions for Biosynthetic Pathway Research.
| Research Reagent / Tool | Function / Application |
|---|---|
| PacBio Long-Read Sequencing | Generates long, continuous DNA reads for improved genome assembly continuity [2]. |
| Hi-C Sequencing | Provides proximity ligation data for scaffolding contigs into chromosome-level assemblies [2]. |
| Bionano Optical Mapping | Creates genome-wide physical maps for hybrid scaffolding and validation of sequence assemblies [2]. |
| Single-Nucleus RNA-seq (sn-RNAseq) | Resolves transcriptomes of individual cells/nuclei, revealing cell-type-specific expression in complex tissues [5]. |
| Heterologous Host (N. benthamiana) | A plant-based system for transiently expressing multiple candidate genes and functionally characterizing enzymes in a live context [5]. |
| Fluorescence In Situ Hybridization (FISH) | Experimentally validates genome assembly accuracy and contig orientation using physical mapping [2]. |
| BioNavi-NP Software | A deep learning toolkit for predicting biosynthetic pathways via retrosynthetic analysis [3]. |
| PlantClusterFinder Pipeline | Identifies biosynthetic gene clusters (BGCs) in plant genomes [2]. |
Defining biosynthetic pathways is a complex but critical endeavor that bridges fundamental science and applied drug discovery. The integration of computational methods, including AI and genome mining, with sophisticated experimental workflows built on multi-omics and heterologous expression, has dramatically accelerated the pace of pathway elucidation. As demonstrated by the recent work on paclitaxel and camptothecin, these integrated strategies are unlocking the potential for synthetic biology to produce high-value natural products in a sustainable and economically viable manner. This progress promises to overcome long-standing supply bottlenecks and opens new frontiers in the development of plant-derived pharmaceuticals.
This case study examines the independent biosynthetic pathways of ipecac alkaloids in two evolutionarily distant medicinal plants, Carapichea ipecacuanha (Gentianales) and Alangium salviifolium (Cornales). Through comparative metabolomics, transcriptomics, and functional enzymology, researchers have elucidated how these species convergently evolved pathways to produce identical medicinally significant compounds—primarily emetine and cephaeline—despite utilizing distinct starting substrates and enzyme suites. The findings provide a model system for understanding the evolution of complex plant natural product pathways and offer a foundation for metabolic engineering approaches to produce these pharmaceutically valuable compounds.
Plant natural products often exhibit lineage-specific distribution, yet there are remarkable instances where identical complex molecules are produced by distantly related species [6]. Ipecac alkaloids represent a pharmaceutically significant example of this phenomenon, with the tetrahydroisoquinoline alkaloids emetine and cephaeline serving as principal bioactive components in traditional and modern medicine [7] [8]. Ipecac syrup, prepared from C. ipecacuanha rhizomes, was historically used as an emetic, while A. salviifolium (sage-leaved alangium or Ankol) finds application in Ayurvedic medicine for similar purposes [6] [9].
What makes these alkaloids particularly intriguing from a biosynthetic perspective is their occurrence in two plant families—Rubiaceae (C. ipecacuanha) and Cornaceae (A. salviifolium)—that diverged approximately 150 million years ago [6]. This evolutionary distance presents a compelling natural experiment for investigating whether nature has arrived at the same complex chemical outcomes through identical or divergent biosynthetic strategies. Recent research has revealed that these plants employ unexpectedly different precursors and enzymes to synthesize the same protoemetine-derived alkaloids, offering unprecedented insights into pathway evolution and enabling future bioengineering of these medicinally important compounds [6] [7] [8].
Background and Significance
Historical Context and Medicinal Importance
Ipecacuanha alkaloids have a long history of medicinal use, particularly as emetics and expectorants, and later as treatments for amebic dysentery [10]. The central importance of emetine and cephaeline as the active emetic principles has been established for decades, though the biosynthetic routes remained largely enigmatic until recent technological advances in genomics and metabolomics [6] [8]. Beyond their emetic properties, certain protoemetine-derived alkaloids like tubulosine exhibit promising anticancer and antimalarial activities, though their low natural abundance has hampered detailed pharmacological investigation [6] [8].
Chemical Structures and Diversity
Ipecac alkaloids are characterized by their monoterpenoid-tetrahydroisoquinoline skeleton, formed through condensation of a monoterpene precursor (secologanin or secologanic acid) with dopamine [6] [9]. The pathway produces multiple stereoisomers and derivatives through modifications including O-methylation, deglycosylation, reduction, and decarboxylation, creating a diverse array of structurally related compounds with varying biological activities [11].
Comparative Analysis of Ipecac Alkaloid Biosynthesis
Pathway Initiation Through Nonenzymatic Chemistry
A fundamental discovery in ipecac alkaloid biosynthesis is the nonenzymatic nature of the initial Pictet-Spengler reaction that couples dopamine with either secologanin (C. ipecacuanha) or secologanic acid (A. salviifolium) [6] [9].
Experimental Evidence for Nonenzymatic Initiation:
- Infiltration Experiments: When secologanin/secologanic acid and dopamine were infiltrated into Nicotiana benthamiana leaves, both 1S and 1R stereoisomers of the Pictet-Spengler products formed within 24 hours without any enzymatic catalysis [6].
- Endogenous Substrate Testing: Catharanthus roseus flower petals containing endogenous secologanin but lacking ipecac alkaloid pathways produced both stereoisomers when infiltrated with dopamine [6].
- Chemical Rationale: Dopamine is highly activated for the Pictet-Spengler reaction, reacting rapidly and nonstereoselectively under mild acidic conditions like those found in the vacuole [6] [9].
This nonenzymatic initiation explains the presence of both 1R and 1S stereoisomers in both plant species and represents a rare example of a complex biosynthetic pathway beginning with a spontaneous chemical reaction rather than enzyme catalysis [6].
Species-Specific Monoterpene Precursors
A key distinction between the two pathways lies in their monoterpene precursors, as revealed through metabolite profiling:
Table 1: Monoterpene Precursor Specificity in Ipecac Alkaloid Biosynthesis
| Plant Species | Monoterpene Precursor | Initial Condensation Products | Chemical Form |
|---|---|---|---|
| Carapichea ipecacuanha | Secologanin | DAII 4a (1S) and DAI 4b (1R) | Ester form |
| Alangium salviifolium | Secologanic acid | DAIIA 5a (1S) and DAIA 5b (1R) | Acid form |
Metabolite profiling demonstrated that secologanin is exclusively observed in C. ipecacuanha, while secologanic acid is found only in A. salviifolium [6]. This precursor specificity aligns with findings in other Cornales species like Camptotheca acuminata, which also utilizes secologanic acid in alkaloid biosynthesis [6].
Tissue-Specific Localization of Pathway Intermediates
Metabolite profiling revealed distinct tissue distribution patterns of ipecac alkaloids in the two species:
Table 2: Tissue-Specific Accumulation of Ipecac Alkaloids
| Plant Species | High Accumulation Tissues | Low Accumulation Tissues | Key Observations |
|---|---|---|---|
| Carapichea ipecacuanha | Young leaves, rhizomes | Mature tissues | Similar alkaloid amounts in young leaves and rhizomes |
| Alangium salviifolium | Leaf buds (intermediates), roots/bark (cephaeline) | Other tissues | Pathway intermediates to protoemetine in leaf buds; cephaeline in roots/older stem bark |
These tissue-specific accumulation patterns guided transcriptome analysis by focusing RNA sequencing on tissues with high alkaloid production, enabling more efficient identification of biosynthetic gene candidates [6].
Divergent Metabolic Fate of Stereoisomers
Following the nonenzymatic Pictet-Spengler reaction, the 1S and 1R stereoisomers undergo species-specific enzymatic processing:
In both species, the 1S-epimer is channeled toward protoemetine through O-methylation, deglycosylation, reduction, and (in C. ipecacuanha) deesterification [6]. The 1R-epimer, however, undergoes different fates: N-acetylation to ipecoside in C. ipecacuanha versus O-methylation to 6-O-Me-DAIA and 7-O-Me-DAIA in A. salviifolium [6] [9].
Experimental Methodologies for Pathway Elucidation
Integrated Metabolomics and Transcriptomics Approach
Researchers employed a comprehensive strategy combining metabolite profiling with gene expression analysis to identify biosynthetic genes:
Experimental Workflow:
- Tissue Selection and RNA Sequencing: Collected high- and low-alkaloid accumulating tissues from both species for RNA-seq library construction [6].
- Metabolite Profiling: Conducted tissue-specific metabolite analysis using LC-MS/MS to identify and quantify pathway intermediates [6].
- Co-expression Analysis: Identified genes whose expression patterns correlated with alkaloid accumulation across tissues [6] [10].
- Functional Characterization: Heterologously expressed candidate genes in model systems (N. benthamiana) and characterized enzyme activities in vitro [6].
This integrated approach successfully identified key biosynthetic genes, including O-methyltransferases and glucosidases, in both species [6].
Key Enzyme Functional Characterization
Several classes of enzymes play critical roles in ipecac alkaloid biosynthesis:
Table 3: Key Enzymes in Ipecac Alkaloid Biosynthesis
| Enzyme Class | Specific Examples | Function in Pathway | Species | Notable Characteristics |
|---|---|---|---|---|
| O-Methyltransferases (OMTs) | IpeOMT1, IpeOMT2, IpeOMT3 [11] | Methylate hydroxy groups on isoquinoline skeleton | C. ipecacuanha | Sufficient for all O-methylation reactions; related to flavonoid OMTs |
| β-Glucosidases | IpeGlu1 [12] | Hydrolyzes glucosidic ipecac alkaloids | C. ipecacuanha | Lacks stereospecificity; prefers 1R-epimers |
| Novel Sugar-Cleaving Enzyme | Not named [7] | Cleaves sugar molecule from alkaloid intermediate | A. salviifolium | Unusual 3D structure; localized in cell nucleus |
The discovery of a sugar-cleaving enzyme localized in the cell nucleus, while its substrate resides in the vacuole, reveals a sophisticated defense strategy where toxic compounds are only produced when herbivory disrupts cellular compartmentalization [7] [8].
Phylogenetic Analysis of Biosynthetic Enzymes
Phylogenetic comparisons of the identified enzymes from both species provide compelling evidence for independent pathway evolution. The O-methyltransferases and other biosynthetic enzymes from C. ipecacuanha and A. salviifolium cluster separately in phylogenetic trees, indicating they were recruited from different ancestral genes rather than inherited from a common ancestor [6]. This pattern of parallel and convergent enzyme evolution explains how both species arrived at the same metabolic outcomes through different molecular mechanisms [6] [9].
Research Reagents and Experimental Tools
Table 4: Essential Research Reagents for Ipecac Alkaloid Studies
| Reagent/Solution | Function/Application | Specific Examples |
|---|---|---|
| Plant Materials | Source of alkaloids and biosynthetic genes | C. ipecacuanha rhizomes and young leaves; A. salviifolium roots and leaf buds [6] |
| Heterologous Expression Systems | Functional characterization of candidate genes | Nicotiana benthamiana infiltration [6]; Model plant transformation [7] |
| Chemical Standards | Metabolite identification and quantification | Secologanin, secologanic acid, dopamine, protoemetine, cephaeline, emetine [6] |
| RNA-seq Tools | Transcriptome analysis and gene discovery | cDNA library construction from high-alkaloid tissues; co-expression analysis [6] [10] |
| Enzyme Assay Components | In vitro functional characterization | Recombinant enzymes, synthetic substrates, AdoMet (for OMTs) [11] |
Implications and Future Directions
Evolutionary Significance
The independent evolution of ipecac alkaloid biosynthesis in C. ipecacuanha and A. salviifolium represents a striking example of convergent metabolic evolution [6] [7]. This system demonstrates how nature can arrive at identical complex chemical outcomes through different biosynthetic strategies, providing insights into the evolutionary mechanisms that generate chemical diversity in plants. The recruitment of different enzyme classes for the same biochemical function suggests inherent flexibility in metabolic evolution and provides a model system for studying how new pathways emerge and become optimized [6].
Biotechnology and Metabolic Engineering Applications
Elucidation of the ipecac alkaloid pathway opens avenues for bioproduction of these medicinally valuable compounds [6] [8]. The limited natural availability of some ipecac alkaloids like tubulosine has hampered pharmacological investigation of their promising anticancer and antimalarial activities [7]. With the genes and enzymes now identified, metabolic engineering in heterologous hosts such as yeast or plants becomes feasible, enabling sustainable production of these compounds for drug development [6] [8].
Unresolved Questions and Research Opportunities
Despite significant advances, important aspects of ipecac alkaloid biosynthesis remain unresolved:
- Final Steps to End Products: The complete pathway has been elucidated only up to protoemetine; the enzymatic steps converting protoemetine to cephaeline, emetine, and tubulosine remain unknown [7].
- Transport and Compartmentalization: The mechanisms controlling subcellular trafficking of pathway intermediates, particularly between vacuole and cytoplasm, require further investigation [10].
- Regulatory Networks: The transcriptional and post-translational regulation of ipecac alkaloid biosynthesis is completely unexplored.
Addressing these questions will complete our understanding of this fascinating example of convergent evolution and facilitate full harnessing of its biotechnological potential.
The independent evolution of ipecac alkaloid biosynthesis in Carapichea ipecacuanha and Alangium salviifolium exemplifies nature's capacity to arrive at identical complex metabolic outcomes through different biochemical strategies. This case study highlights how nonenzymatic chemistry can initiate specialized metabolic pathways and how spatial organization of enzymes and substrates enables production of toxic defense compounds. The findings provide both fundamental insights into metabolic evolution and practical foundations for engineering production of these medically valuable compounds. As a model system, ipecac alkaloid biosynthesis continues to offer rich opportunities for exploring the principles governing the emergence and optimization of plant natural product pathways.
The elucidation of biosynthetic pathways represents a cornerstone of synthetic biology and metabolic engineering, with profound implications for drug discovery, sustainable production of natural products, and fundamental understanding of biological systems. Despite tremendous advances in sequencing technologies and computational tools, significant challenges persist in bridging the gap between genetic information and functional metabolic pathways. The vast universe of enzymatic functions remains largely unexplored, with current knowledge likely representing only a fraction of nature's true biocatalytic diversity [13]. This whitepaper examines the key challenges facing researchers in the field of biosynthetic pathway discovery, focusing specifically on the hurdles from uncharacterized enzyme functions to incomplete pathway knowledge, while providing technical guidance and methodological frameworks to address these obstacles.
The scale of the challenge becomes apparent when considering the vast data resources available to researchers. Structural protein databases such as UniProt now contain more than 227 million protein sequences, while the Protein Data Bank archives over 180,000 three-dimensional protein structures, with more than 200 million additional structures predicted computationally [13]. However, the functional annotation of these sequences has not kept pace with their discovery, creating an ever-widening sequence-function gap that represents one of the most significant bottlenecks in biosynthetic pathway elucidation. Within this landscape, researchers must navigate the complexities of enzyme promiscuity, stereochemical diversity, metabolic network robustness, and the limitations of both experimental and computational methods for function prediction.
The Sequence-Function Gap: Navigating the Unknown Proteome
The Scale of the Annotation Challenge
The fundamental challenge in connecting protein sequences to their biological functions stems from the limitations of annotation transfer based on sequence similarity. While over 80% of entries in the UniProt database are assigned to at least one Pfam or InterPro family, these assignments often provide only tentative functional descriptions that may not reflect the true in vivo activities of these proteins [14]. The problem is compounded by the natural evolutionary process whereby gene duplication events followed by functional diversification create families of structurally similar enzymes with distinct biological functions [15]. This divergence means that sequence similarity alone is insufficient for accurate function prediction, as demonstrated by cases where enzymes with high structural similarity exhibit dramatically different catalytic efficiencies—sometimes varying by up to four orders of magnitude despite their evolutionary relationship [15].
The limitations of current computational approaches were starkly illustrated in a case study where a transformer deep learning model trained on 22 million enzymes made hundreds of erroneous "novel" predictions, including assigning mycothiol synthase activity to an E. coli gene despite mycothiol not being synthesized by E. coli at all [15]. Of 450 novel predictions made by the model, 135 were already listed in the database used for training and thus not actually novel, while 148 showed biologically implausible levels of repetition with the same highly specific functions reappearing up to 12 times for E. coli genes [15]. These errors highlight the critical limitations of supervised machine learning for discovering truly unknown functions, as noted by domain experts: "By design, supervised ML-models cannot be used to predict the function of true unknowns" [15].
Methodologies for Functional Annotation
To address the sequence-function gap, researchers have developed genomic enzymology tools that integrate multiple lines of evidence for more robust function prediction. The Enzyme Function Initiative (EFI) provides a suite of freely accessible tools that have been used in over 300 publications to date [14]. The two primary components are:
EFI-EST (Enzyme Similarity Tool): Generates sequence similarity networks (SSNs) for protein families using all-by-all pairwise BLAST comparisons. In an SSN, each sequence is represented as a node, with edges connecting nodes that share user-specified sequence similarity thresholds. As the similarity threshold increases, the network segregates into isofunctional clusters that can be annotated with known functions or identified as targets for novel function discovery [14].
EFI-GNT (Genome Neighborhood Tool): Generates genome neighborhood networks (GNNs) and diagrams (GNDs) that provide metabolic context for proteins of interest. In bacterial, archaeal, and fungal genomes, operons and gene clusters often encode functionally linked enzymes in metabolic pathways, allowing researchers to make informed hypotheses about function based on genomic context [14].
Table 1: Genomic Enzymology Tools for Functional Annotation
| Tool | Methodology | Application | Key Output |
|---|---|---|---|
| EFI-EST | Sequence similarity networks (SSNs) | Visualizing sequence-function space in protein families | Isofunctional clusters of uncharacterized enzymes |
| EFI-GNT | Genome neighborhood networks (GNNs) | Identifying metabolically linked genes in pathways | Hypotheses about metabolic pathway participation |
| SSN/GNN Integration | Combined sequence and context analysis | Robust functional prediction for uncharacterized clusters | Testable hypotheses for experimental validation |
The practical application of these tools is illustrated by their use in exploring the diheme peroxidase family (Pfam PF03150). SSN analysis revealed several uncharacterized clusters emerging from known functions, including a cluster (IIIb) from Bulkholderia genomes that lacked the methylamine dehydrogenase typically associated with this family [14]. Subsequent characterization showed that while these enzymes could reduce H₂O₂ to H₂O like typical cytochrome c peroxidases, they also generated a bis-Fe(IV) species found on the reaction coordinate of the structurally related but mechanistically distinct MauG enzyme [14]. This example demonstrates how SSN analysis can identify enzymes with novel functional properties that would be missed by simple sequence similarity searches.
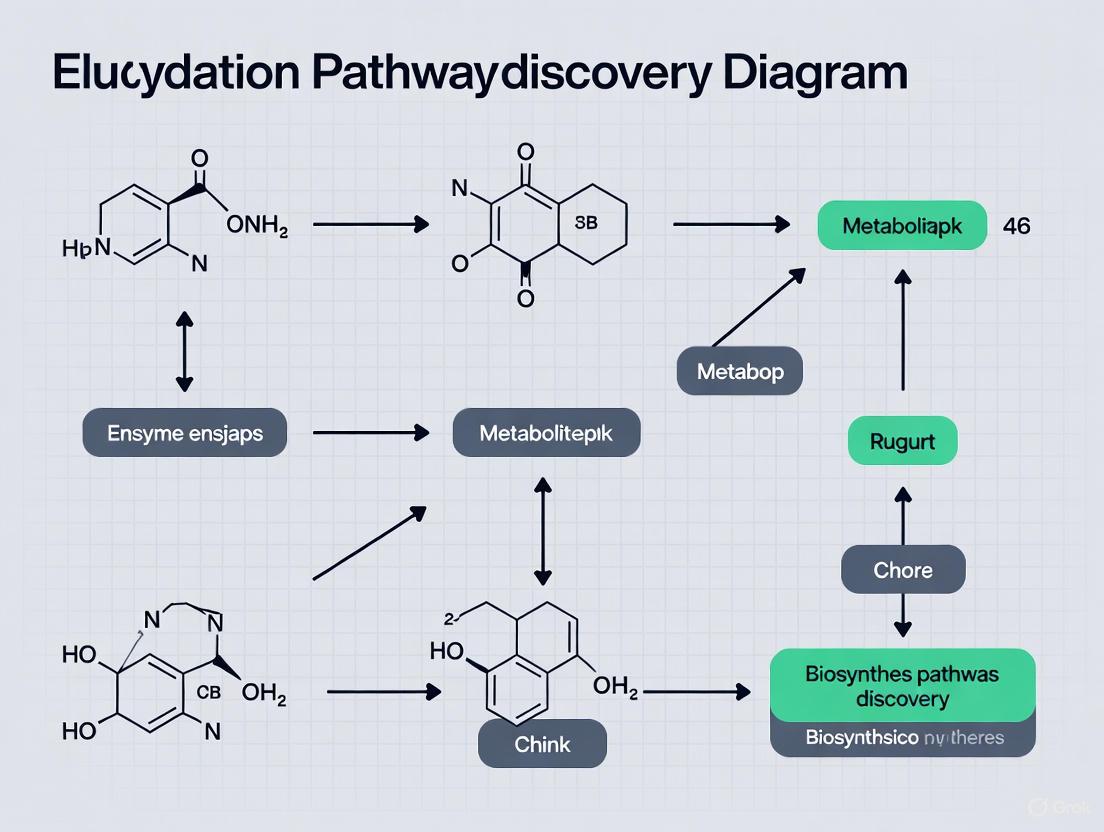
Figure 1: Genomic Enzymology Workflow for Novel Enzyme Discovery
Challenges in Pathway Elucidation and Reconstruction
Navigating Complex Metabolic Spaces
The reconstruction of complete biosynthetic pathways faces multiple challenges, including massive search spaces, complex metabolic interactions, and biological system uncertainties [16]. Traditional approaches to pathway discovery require extensive experimental effort, with notable examples including the 150 person-years needed to elucidate the artemisinin precursor pathway and 575 person-years for propanediol [16]. The complexity arises from several factors: the combinatorial explosion of possible pathway combinations, the presence of promiscuous enzymes that can accept multiple substrates, and the compartmentalization of metabolic pathways within cells that is difficult to recapitulate in heterologous systems.
Plant natural products exemplify these challenges, as their biosynthetic pathways often involve complex regulation, gene clusters, protein complexes (metabolons), and transport processes that are not fully understood [17]. Strategies to address these complexities include co-expression analysis, gene cluster identification, metabolite profiling, deep learning approaches, genome-wide association studies, and protein complex identification [17]. However, each of these methods has limitations, and integrated approaches are typically required for successful pathway elucidation.
Computational Tools for Pathway Prediction
Computational methods have become indispensable for navigating the complex landscape of biosynthetic pathway discovery. These tools can be broadly categorized into knowledge-based and rule-based approaches, with recent advances incorporating deep learning methodologies [3]. Knowledge-based approaches enumerate possible biosynthesis routes according to existing reaction databases such as MetaCyc and KEGG, ranking suggested routes through scoring functions including chemical similarity and chassis compatibility [3]. However, these methods fail for complex natural products whose biosynthetic reactions are not represented in existing databases.
Rule-based models match query molecules to generalized reaction rules—subgraph patterns that highlight changes during biochemical reactions. Tools like RetroPath2.0 and RetroPathRL use these rules to propose potential biosynthetic routes [3]. While promising, these approaches face challenges in formulating expert-approved rules, determining appropriate rule generality/specificity, and their fundamental inability to predict reactions beyond existing rule databases [3].
Recent advances in deep learning have enabled rule-free prediction models that show superior performance and generalization potential. BioNavi-NP is one such tool that uses transformer neural networks trained on both general organic and biosynthetic reactions to predict biosynthetic pathways through an AND-OR tree-based planning algorithm [3]. The system achieves a top-10 prediction accuracy of 60.6% on single-step biosynthetic test sets, 1.7 times more accurate than conventional rule-based approaches, and can identify biosynthetic pathways for 90.2% of test compounds [3].
Table 2: Computational Tools for Biosynthetic Pathway Prediction
| Tool/Method | Approach | Advantages | Limitations |
|---|---|---|---|
| Knowledge-Based | Uses existing reaction databases (MetaCyc, KEGG) | Biologically relevant predictions | Limited to known reactions in databases |
| Rule-Based (RetroPath2.0, RetroPathRL) | Matches molecular subgraph patterns | Can propose novel combinations of known reactions | Limited by quality and coverage of reaction rules |
| Deep Learning (BioNavi-NP) | Transformer neural networks trained on reaction data | High accuracy and generalization potential | Requires substantial training data, limited interpretability |
| Hybrid Approaches | Combines multiple methods and data sources | Leverages strengths of different approaches | Implementation complexity |
Experimental Methodologies for Pathway Validation
Integrated Computational-Experimental Workflows
The validation of predicted enzyme functions and biosynthetic pathways requires carefully designed experimental workflows that integrate computational predictions with laboratory verification. A robust approach begins with computational prediction followed by multiple stages of experimental validation:
Gene Identification and Synthesis: Candidate genes identified through SSN analysis or pathway prediction tools are synthesized or cloned for expression. For enzymes with potential industrial or therapeutic applications, miniaturization strategies may be employed to enhance expression, folding efficiency, and stability [18]. Enzyme miniaturization has been shown to improve thermostability, resistance to proteolysis, and interfacial electron transfer rates in biosensors [18].
Protein Expression and Purification: Selected genes are expressed in suitable host systems (typically E. coli or yeast) and proteins are purified using affinity chromatography. Smaller enzymes (<200 amino acids) generally exhibit higher expression yields and superior folding efficiency compared to larger proteins, which often require fusion tags or chaperones for soluble expression [18].
In Vitro Activity Assays: Purified enzymes are tested for predicted activities using appropriate substrates. Kinetic parameters (kcat and Km) are determined to quantify catalytic efficiency and substrate specificity. The SKiD (Structure-oriented Kinetics Dataset) provides a curated resource of enzyme-substrate interactions with associated kinetic parameters and structural information to support these analyses [19].
In Vivo Functional Validation: For pathway validation, candidate genes are introduced into microbial hosts to test for production of target compounds. Metabolite profiling using LC-MS or GC-MS confirms the presence of expected pathway intermediates and products.
Structural Characterization: When possible, enzyme structures are determined through X-ray crystallography or cryo-EM to provide mechanistic insights and guide further engineering.
Structural Kinetics in Enzyme Characterization
The integration of structural information with kinetic data provides powerful insights into enzyme function and mechanism. The SKiD dataset represents a significant advance in this area, containing 13,653 unique enzyme-substrate complexes with associated kinetic parameters (kcat and Km) and structural information [19]. This resource enables researchers to correlate structural features with catalytic efficiency, informing enzyme engineering efforts.
For example, studies of serine proteases have demonstrated how the precise spatial arrangement of catalytic triad residues (Ser, His, Asp) determines substrate specificity and catalytic efficiency [19]. Similarly, analysis of the E. coli haloacid dehalogenase-like hydrolase superfamily showed better correlation between catalytic efficiency and structural features than with sequence similarity alone [19]. These findings underscore the importance of structural data in understanding and engineering enzyme function.
Figure 2: Integrated Pathway Discovery and Validation Workflow
Successful navigation of the challenges in biosynthetic pathway elucidation requires leveraging a diverse array of databases, computational tools, and experimental resources. The table below summarizes key resources available to researchers in the field.
Table 3: Essential Research Resources for Biosynthetic Pathway Elucidation
| Resource Category | Specific Tools/Databases | Key Features and Applications |
|---|---|---|
| Compound Databases | PubChem, ChEBI, ChEMBL, ZINC, ChemSpider | Chemical structures, properties, and bioactivity data for substrate identification |
| Reaction/Pathway Databases | KEGG, MetaCyc, Reactome, Rhea, SABIO-RK | Curated metabolic pathways and enzyme-catalyzed reactions for pathway reconstruction |
| Enzyme Databases | UniProt, BRENDA, PDB, AlphaFold DB | Protein sequences, functions, mechanisms, and structural information |
| Genomic Enzymology Tools | EFI-EST, EFI-GNT | Sequence similarity networks and genome neighborhood analysis for function prediction |
| Pathway Prediction Tools | BioNavi-NP, RetroPath2.0, RetroPathRL | Deep learning and rule-based approaches for biosynthetic route planning |
| Kinetic Data Resources | SKiD, BRENDA, SABIO-RK | Enzyme kinetic parameters (kcat, Km) for pathway modeling and optimization |
| Specialized NP Databases | NPAtlas, LOTUS, COCONUT, NPASS | Curated natural product structures with biosynthetic and bioactivity information |
The elucidation of complete biosynthetic pathways remains a formidable challenge at the intersection of genomics, enzymology, and metabolic engineering. The key obstacles—from the sequence-function gap to incomplete pathway knowledge—require integrated computational and experimental approaches that leverage the growing arsenal of databases, prediction tools, and characterization methods. While significant challenges remain, advances in genomic enzymology tools, deep learning approaches, and structural kinetics are progressively enabling researchers to navigate the complex landscape of biosynthetic pathway discovery.
The field is moving toward more sophisticated integration of multi-omics data, AI-driven prediction, and automated experimental validation workflows. As these technologies mature, they promise to accelerate the discovery and engineering of biosynthetic pathways for natural products and other valuable compounds, with profound implications for drug discovery, sustainable manufacturing, and fundamental understanding of biological systems. However, as the limitations of purely computational approaches demonstrate, domain expertise and careful experimental validation remain essential for robust pathway elucidation. The future of biosynthetic pathway discovery lies in the continued integration of computational power with deep biological insight, enabling researchers to bridge the gap from sequence to function to complete pathway understanding.
The Role of Plant Natural Products as a Source of Medicines and Agrochemicals
Plant natural products (PNPs), also known as specialized metabolites, constitute a cornerstone of both traditional and modern therapeutics, serving as a major reservoir for drug discovery and development. Over one-third of FDA-approved drugs are derived from natural products and their derivatives [20]. These compounds, such as the anticancer drugs topotecan (from camptothecin) and etoposide (from podophyllotoxin), showcase the immense pharmaceutical value of plant chemical diversity [21]. However, the full potential of PNPs is hindered by the complexity of their biosynthetic pathways, which often remain only partially understood. This whitepaper delineates the contemporary strategies and methodologies propelling the elucidation of these pathways, framing them within the critical context of biosynthetic pathway discovery research. The integration of multi-omics technologies, advanced computational tools, and innovative functional characterization techniques is transforming this field, enabling researchers to unravel intricate metabolic networks and paving the way for the sustainable bioproduction of valuable plant-derived medicines and agrochemicals [17] [21].
The Therapeutic Significance of Plant Natural Products
Plants produce an enormous reservoir of chemicals, estimated to encompass over one million specialized metabolites, which play vital eco-physiological roles in plant adaptation and possess a wide array of therapeutic bioactivities [21]. The significance of PNPs in modern medicine is demonstrated by numerous clinically important derivatives. For instance, vinblastine, used to treat Hodgkin's lymphoma, is derived from the Madagascar periwinkle (Catharanthus roseus), and the antimalarial compound artemisinin is isolated from sweet wormwood (Artemisia annua) [21]. The table below summarizes key plant-derived drugs and their therapeutic applications.
Table 1: Clinically Important Drugs Derived from Plant Natural Products
| Drug Name | Plant Natural Product Origin | Therapeutic Application | Biosynthetic Status |
|---|---|---|---|
| Topotecan | Camptothecin (from Camptotheca acuminata) | Anticancer | Pathway partially elucidated; key enzymes like OpCYP716E111 identified [21] [22] |
| Etoposide | Podophyllotoxin (from Podophyllum species) | Anticancer | Biosynthetic pathway discovery accelerated by co-expression analysis [21] |
| Vinblastine/Vincristine | Precursors from Catharanthus roseus | Anticancer | Complete biosynthetic pathway elucidated [21] |
| Morphine | Codeinone (from Papaver somniferum) | Analgesic | Complete biosynthetic pathway elucidated [21] |
| Noscapine | (from Papaver somniferum) | Antitussive, Anticancer | Complete biosynthetic pathway elucidated [21] |
| HSYA (Investigational) | (from Carthamus tinctorius - Safflower) | Acute Ischemic Stroke | Pathway recently elucidated involving CtCGT, CtF6H, Ct2OGD1, CtCHI1 [23] |
A contemporary example of a PNP with significant clinical promise is Hydroxysafflor yellow A (HSYA) from safflower (Carthamus tinctorius). HSYA is a unique quinochalcone di-C-glycoside that has completed a phase III clinical trial for the treatment of acute ischemic stroke in China [23]. Its complex structure had made total chemical synthesis a great challenge, highlighting the necessity of elucidating its biosynthetic pathway for sustainable production [23].
Core Strategies for Biosynthetic Pathway Elucidation
The process of decoding a plant's biosynthetic pathway is a multi-stage endeavor that integrates genomics, transcriptomics, metabolomics, and functional validation. The following workflow visualizes the core logical process and data integration points in a modern pathway elucidation pipeline.
Multi-Omics Guided Discovery
The advent of next-generation sequencing has revolutionized pathway discovery by generating comprehensive omics datasets [21]. The core bioinformatic approaches for candidate gene identification include:
- Co-expression Analysis: This method identifies genes whose expression patterns correlate across different tissues, organs, or treatments with the accumulation of the target metabolite or with known pathway genes. For example, the elucidation of the colchicine and strychnine pathways relied heavily on Pearson correlation-based co-expression analysis [21]. Tools like self-organizing maps have also been successfully applied for vinblastine and camptothecin [21].
- Homology-Based Identification: This strategy leverages evolutionary relationships to find enzymes. Using tools like OrthoFinder, researchers can identify genes homologous to those encoding enzymes that catalyze similar reactions in other species. This approach was instrumental in discovering enzymes for spiroxindole and benzylisoquinoline alkaloid pathways [21].
- Gene Cluster Identification: Although less common in plants than in microbes, biosynthetic genes are sometimes co-localized in the genome. Genomic proximity can, therefore, be a powerful predictor of pathway membership [17] [21].
- Genome-Wide Association Studies (GWAS): GWAS links genetic variants to metabolic traits, helping pinpoint genomic regions associated with the production of specific natural products [17].
Emerging Techniques: Chemoproteomics
Chemoproteomics has emerged as a powerful functional tool that complements omics-based predictions. It uses designed chemical probes to directly isolate and identify active enzymes from complex plant proteomes, bypassing some limitations of traditional genetics-based methods [22].
Workflow of Affinity Probes:
- Probe Design: A small-molecule probe is synthesized, typically containing three key elements:
- A binding moiety that mimics the natural enzyme substrate.
- A reactive group (e.g., a diazirine) that forms a covalent bond with the enzyme upon photoactivation.
- A reporter tag (e.g., biotin) for detection and purification of the enzyme-probe complex.
- Probe Incubation and Activation: The probe is incubated with a native plant protein extract. Photoactivation cross-links the probe to enzymes that have a specific affinity for the substrate mimic.
- Purification and Identification: The tagged enzyme-probe complexes are captured (e.g., using streptavidin beads) and the enzymes are identified via mass spectrometry [22].
Key Applications:
- Steviol Glycosides: Chemoproteomics identified specific UDP-glycosyltransferases (UGTs) responsible for the glycosylation of steviol in Stevia rebaudiana [22].
- Camptothecin: A diazirine-based probe specific to strictosamide helped identify the long-sought epoxidase OpCYP716E111 in the camptothecin pathway [22].
- Chalcomoracin: This approach led to the discovery of a novel Diels-Alderase (MaDA) in mulberry, which catalyzes a key cycloaddition reaction [22].
Detailed Experimental Protocols for Key Methods
Heterologous Expression and Functional Characterization
This protocol is central to validating the function of candidate enzymes identified through bioinformatics.
Objective: To express a candidate plant gene in a heterologous host and assay its enzymatic activity.
Materials & Reagents:
- Cloning System: Expression vectors (e.g., pET for E. coli, pYES2 for yeast).
- Heterologous Hosts: Escherichia coli (BL21), Saccharomyces cerevisiae (yeast), or Nicotiana benthamiana (tobacco) for transient expression.
- Culture Media: LB for E. coli, appropriate selective media for yeast, and agroinfiltration media for N. benthamiana.
- Substrates: Putative enzymatic substrate (e.g., naringenin for CtF6H [23]).
- Analytical Tools: Liquid Chromatography-Mass Spectrometry (LC-MS).
Procedure:
- Gene Cloning: Amplify the candidate gene's coding sequence and clone it into an appropriate expression vector.
- Transformation: Introduce the recombinant vector into the heterologous host.
- For E. coli: Use chemical transformation or electroporation.
- For N. benthamiana: Use Agrobacterium tumefaciens-mediated transient transformation (agroinfiltration) [21].
- Protein Expression: Induce gene expression (e.g., with IPTG for E. coli or galactose for yeast). For N. benthamiana, incubate plants for 2-4 days post-infiltration.
- Protein Extraction: Lyse cells to extract proteins. For membrane-bound P450s (like CtF6H [23]), isolate microsomal fractions from yeast or N. benthamiana.
- In Vitro Enzyme Assay:
- Set up a reaction mixture containing the extracted protein, the putative substrate, and necessary cofactors (e.g., NADPH for P450s, UDP-glucose for glycosyltransferases).
- Incubate at the optimal temperature and pH (e.g., CtCGT showed maximum activity at pH 9.0 and 45°C, while CtF6H was most active at 4°C [23]).
- Terminate the reaction and extract metabolites.
- Product Analysis: Analyze the reaction products using LC-MS. Compare the chromatograms and mass spectra to those of controls and authentic standards to confirm the formation of the expected product.
Virus-Induced Gene Silencing (VIGS) in Planta
Objective: To knock down the expression of a candidate gene in the native plant host and observe the metabolic consequences, thereby confirming its physiological role.
Materials & Reagents:
- VIGS Vector: A TRV (Tobacco Rattle Virus)-based vector system.
- Agrobacterium tumefaciens: Strain GV3101.
- Plant Material: Young seedlings of the target plant (e.g., safflower [23]).
Procedure:
- Vector Construction: Clone a 300-400 bp fragment specific to the target gene (e.g., CtCGT or CtF6H [23]) into the VIGS vector.
- Agrobacterium Preparation: Transform the recombinant VIGS vector into Agrobacterium. Grow cultures to an OD600 of ~1.0.
- Plant Infiltration: Infiltrate the Agrobacterium suspension into the leaves of young seedlings (e.g., two-week-old safflower seedlings [23]) using a needleless syringe.
- Growth and Monitoring: Grow plants for a sufficient period to allow gene silencing and metabolite turnover (e.g., ~2 months for safflower to reach the budding stage [23]).
- Metabolite and Gene Expression Analysis:
- Harvest tissues (e.g., flowers) and analyze the content of the target metabolite (e.g., HSYA) via LC-MS.
- Simultaneously, quantify the expression level of the silenced gene using quantitative RT-PCR.
- Validation: A successful VIGS experiment shows a significant reduction in both the target metabolite and the transcript level of the candidate gene compared to empty-vector control plants, as demonstrated in the elucidation of the HSYA pathway [23].
The Scientist's Toolkit: Essential Research Reagents and Solutions
Successful pathway elucidation relies on a suite of specialized reagents and platforms. The following table details key solutions used in the featured experiments and the broader field.
Table 2: Key Research Reagent Solutions for Biosynthetic Pathway Elucidation
| Reagent / Solution | Category | Function & Application |
|---|---|---|
| Nicotiana benthamiana | Heterologous Host | Used for Agrobacterium-mediated transient expression; allows rapid, simultaneous co-expression of multiple genes for pathway reconstitution [21]. |
| pEAQ-series Vectors | Expression Vector | Plant expression vectors designed for high-level transient expression in N. benthamiana. |
| WAT11 Yeast Strain | Heterologous Host | Engineered Saccharomyces cerevisiae strain expressing a plant P450 reductase, optimized for functional expression of plant cytochrome P450 enzymes [23]. |
| TRV-based VIGS Vectors | Functional Genomics | Virus-Induced Gene Silencing vectors used to knock down gene expression in planta to confirm gene function [21] [23]. |
| Activity-Based Probes (e.g., Diazirine-based) | Chemoproteomics | Designed chemical probes that covalently bind to active site of enzymes, enabling their purification and identification from complex proteomes [22]. |
| UDP-Glucose | Enzyme Assay Reagent | Sugar donor substrate for glycosyltransferase assays (e.g., used with CtCGT [23]). |
| NADPH Regeneration System | Enzyme Assay Reagent | Provides essential cofactor for cytochrome P450 (e.g., CtF6H [23]) and other oxidoreductase enzymes. |
| LC-MS/MS Systems | Analytical Equipment | Used for metabolite profiling, identification, and quantification throughout the discovery and validation process [23]. |
Case Study: De Novo Biosynthesis of Hydroxysafflor Yellow A (HSYA)
The recent elucidation of the HSYA pathway in safflower (Carthamus tinctorius) provides a quintessential example of the integrated application of these modern strategies [23]. The complete biosynthetic pathway, from the central precursor naringenin to HSYA, was decoded and reconstructed.
Pathway Elucidation Steps:
- Proposal and Omics Data Generation: Based on the structure of HSYA, a biosynthetic route from naringenin chalcone was proposed. Transcriptome and metabolome data were generated from different safflower tissues (budding flower, blooming flower, calyx, leaf) [23].
- Candidate Gene Identification: Co-expression analysis using chalcone synthase (CtCHS2) as bait identified candidate genes. This highlighted a cytochrome P450 (CtF6H) and a glycosyltransferase (CtCGT) highly expressed in HSYA-accumulating budding flowers [23].
- Functional Validation:
- In Vitro Assays: CtCGT was expressed in E. coli and shown to catalyze the di-C-glycosylation of 2-hydroxynaringenin. CtF6H was expressed in WAT11 yeast and demonstrated to perform the 6-hydroxylation of naringenin to form carthamidin [23].
- VIGS: Silencing CtCGT or CtF6H in safflower flowers led to a ~30% reduction in HSYA content, confirming their roles in vivo [23].
- Pathway Completion: Two additional enzymes were characterized: a chalcone-flavanone isomerase (CtCHI1) and a 2-oxoglutarate-dependent dioxygenase (Ct2OGD1). The coordinated action of CtCGT and Ct2OGD1 was found to be crucial for converting the flavanone precursors into the final quinochalcone structure of HSYA [23].
- Reconstitution: The entire pathway was successfully reconstituted in Nicotiana benthamiana, achieving de novo biosynthesis of HSYA in a heterologous host [23].
The elucidation of plant natural product biosynthetic pathways is no longer solely reliant on serendipity and labor-intensive biochemistry. The field has been transformed by a powerful, integrated methodology that leverages big data from multi-omics technologies and interrogates it with advanced computational analytics and innovative functional genomics and proteomics tools [17] [21]. As demonstrated by the successful decoding of pathways for complex molecules like HSYA, vinblastine, and strychnine, this systematic approach is drastically accelerating the pace of discovery.
The future of PNP research lies in further refining and integrating these technologies. Key directions include:
- Deep Learning and AI Integration: AI-powered tools will become increasingly critical for predicting enzyme function, metabolic network dynamics, and for de novo design of optimized enzymes and pathways [17] [21].
- Metabolon Engineering: Understanding and engineering enzyme complexes (metabolons) will enhance the efficiency of reconstructed pathways in heterologous hosts [17].
- Single-Cell Omics: Applying transcriptomics and metabolomics at the single-cell level will provide unprecedented resolution of metabolic processes within specific cell types, uncovering further regulatory complexity [21].
- Sustainable Bioproduction: The ultimate application of pathway elucidation is to enable the cheap, green, and scalable production of high-value PNPs through microbial or plant-based synthetic biology, reducing reliance on field cultivation and complex chemical synthesis [17] [22] [20].
By continuing to unravel the intricate biosynthetic networks of plants, researchers not only unlock nature's chemical logic but also establish the foundation for a more sustainable and efficient pipeline for discovering and producing the medicines and agrochemicals of tomorrow.
Multi-Omics and AI: A Next-Generation Toolkit for Pathway Discovery
The elucidation of biosynthetic pathways represents a fundamental challenge in biological research, with significant implications for drug discovery, agricultural science, and synthetic biology. Individual omics technologies—genomics, transcriptomics, and metabolomics—provide valuable but incomplete insights into these complex processes. Genomics offers a blueprint of potential biosynthetic machinery, transcriptomics reveals dynamic gene expression patterns, and metabolomics provides a snapshot of the resulting biochemical phenotypes. However, when integrated within a unified analytical framework, these technologies enable researchers to reconstruct biosynthetic pathways with unprecedented precision and efficiency. This integrated approach is particularly crucial for specialized metabolism in plants, where it is estimated that over 200,000 specialized metabolites play vital roles in adaptation and defense, yet their biosynthetic pathways remain largely uncharacterized [24] [25].
The fundamental challenge in traditional pathway elucidation approaches lies in their requirement for prior knowledge of either a key compound or a critical enzyme, which serves as 'bait' to identify other pathway components [25]. This targeted approach inherently limits discovery to extensions of known pathways, leaving truly novel biosynthetic systems difficult to uncover. Multi-omics integration overcomes this limitation through systematic, unsupervised computational workflows that can predict candidate metabolic pathways de novo by leveraging correlated abundance patterns across molecular layers and knowledge of biochemical reaction rules [24]. This paradigm shift from targeted to discovery-based approaches is accelerating the characterization of biosynthetic pathways for pharmaceutical and agricultural applications.
Core Methodologies and Workflows
Experimental Design and Data Generation
Successful multi-omics integration begins with strategic experimental design that ensures biological congruence across data types. For biosynthetic pathway elucidation, experiments should ideally span a range of different conditions, tissues, and timepoints to capture the dynamic coordination between genes and metabolites [24] [25]. Sample integrity is paramount, particularly for metabolomics where rapid quenching of metabolism is essential to preserve accurate metabolite profiles [26].
Table 1: Sample Processing Methods Across Omics Technologies
| Omics Technology | Sample Collection Considerations | Extraction Methods | Stabilization Approaches |
|---|---|---|---|
| Metabolomics | Type depends on research question (cells, tissue, blood, urine) | Liquid-liquid extraction (e.g., MeOH/CHCl3 for polar/non-polar metabolites), Solid-phase extraction | Flash freezing in liquid N₂, chilled methanol (-20°C to -80°C), ice-cold PBS |
| Transcriptomics | Must preserve RNA integrity; compatible with spatial context preservation | TRIzol, column-based RNA extraction, single-cell encapsulation | RNAlater, rapid freezing, immediate embedding for spatial transcriptomics |
| Genomics | Stable DNA; can use same tissue as other omics with proper partitioning | Phenol-chloroform, commercial DNA kits, magnetic bead-based extraction | Ethanol precipitation, freezing, chelating agents |
For metabolomics, proper sample processing involves rapid quenching of metabolism followed by extraction methods that quantitatively reflect endogenous metabolite levels. Efficient extraction requires optimization based on sample type and the metabolomics strategy (targeted vs. untargeted). Biphasic liquid-liquid extraction using methanol/chloroform/water systems enables simultaneous extraction of polar metabolites (in the methanol/water phase) and non-polar metabolites including lipids (in the chloroform phase) [26]. The addition of internal standards (typically stable isotope-labeled analogs of metabolites) at the beginning of extraction is critical for accurate quantification and quality control [26].
For transcriptomics, emerging spatial technologies preserve architectural context that can be crucial for understanding biosynthetic pathways that occur in specific cell types or tissue regions. These technologies overcome the limitations of traditional bulk RNA sequencing, which averages gene expression across tissues, and even single-cell RNA sequencing, which removes cells from their native spatial context [27]. Platforms such as 10× Visium, Slide-seq V2, and Stereo-seq now provide subcellular-resolution transcriptomic maps while maintaining spatial coordinates, though application in plants presents technical challenges due to rigid cell walls and abundant compounds that inhibit enzymatic reactions [27].
Data Integration and Computational Analysis
The core challenge of multi-omics integration lies in computationally connecting correlated patterns across molecular layers to infer functional relationships. MEANtools represents a significant advancement in this area as a systematic computational workflow that predicts candidate metabolic pathways de novo by leveraging general reaction rules and metabolic structures from public databases [24] [25]. The pipeline implements a mutual rank (MR)-based correlation approach to identify mass features that are highly correlated with biosynthetic genes across samples, then assesses whether observed chemical differences between these metabolites can be explained by reactions catalyzed by transcript-encoded enzyme families [25].
The workflow integrates several key resources: (1) RetroRules, a retrosynthesis-oriented database of enzymatic reactions annotated with protein domains and enzymes; (2) LOTUS, a comprehensive resource of natural products used for putative structural annotation of metabolite features; and (3) MetaNetX, a repository of metabolic networks used to identify mass differences between substrates and products of known enzymatic reactions [25]. This integration enables the construction of a reaction network where nodes represent mass signatures and edges represent enzymatic reactions that can be catalyzed by correlated enzyme families. The database coverage is robust, with RetroRules containing approximately 72% of experimentally characterized biosynthetic reactions from a reference set, significantly higher than expected by chance [25].
Other computational platforms support various aspects of multi-omics integration. MetaboAnalyst provides comprehensive metabolomics data analysis capabilities, including functional analysis of untargeted metabolomics data through its "MS Peaks to Pathways" module, which supports over 120 species [28]. For spatial transcriptomics integration, specialized bioinformatics pipelines are required to address plant-specific challenges, including overcoming limitations posed by rigid cell walls, expansive vacuoles that dilute intracellular content, and abundant polyphenols that inhibit enzymatic reactions [27].
Table 2: Key Computational Tools for Multi-Omics Integration in Pathway Elucidation
| Tool/Platform | Primary Function | Data Types Integrated | Key Features |
|---|---|---|---|
| MEANtools | De novo pathway prediction | Metabolomics, Transcriptomics | Uses reaction rules from RetroRules, structural matching with LOTUS, mutual rank correlation |
| MetaboAnalyst | Metabolomics data analysis and functional interpretation | Metabolomics, Genomics (for joint pathway analysis) | Pathway analysis for >120 species, MS Peaks to Pathways, dose-response analysis |
| PlantiSMASH | Identification of biosynthetic gene clusters | Genomics | Specialized for plant specialized metabolism, detects co-localized biosynthetic genes |
| Spatial Transcriptomics Pipelines | Spatial mapping of gene expression | Transcriptomics (with spatial context), potentially metabolomics | Preservation of tissue architecture, identification of spatially correlated expression |
Machine learning and artificial intelligence are increasingly transforming multi-omics data analysis. AI algorithms have demonstrated improvements in accuracy of up to 30% while reducing processing time by half for some genomics applications [29]. In single-cell transcriptomics, machine learning enables key analytical tasks including clustering, dimensionality reduction, trajectory inference, and cell type annotation [30]. The integration of language models that interpret genetic sequences represents an emerging frontier, with potential to translate nucleic acid sequences to identify patterns and relationships that might be missed by conventional approaches [29].
Practical Implementation and Research Toolkit
Reagents and Materials
Successful multi-omics studies require careful selection of research reagents and materials throughout the workflow. The table below details essential components for integrated genomics, transcriptomics, and metabolomics studies focused on biosynthetic pathway elucidation.
Table 3: Essential Research Reagents and Materials for Multi-Omics Studies
| Category | Specific Reagents/Materials | Function/Purpose | Considerations |
|---|---|---|---|
| Sample Collection & Stabilization | RNAlater, liquid nitrogen, sterile collection containers | Preserve nucleic acid and metabolite integrity | Maintain consistency in collection time/conditions; rapid processing |
| Metabolite Extraction | Methanol, chloroform, MTBE, water (varying ratios) | Extract diverse metabolite classes | Biphasic systems separate polar/non-polar metabolites; pH adjustments can optimize specific classes |
| Internal Standards | Stable isotope-labeled metabolites | Normalization and quality control | Add early in extraction; should represent metabolite classes of interest |
| Nucleic Acid Extraction | TRIzol, column-based kits, magnetic beads | Isolate DNA/RNA | Quality checks (RIN for RNA) essential; compatibility with sequencing platforms |
| Sequencing | Library preparation kits, barcoded adapters, enzymes | Prepare sequencing libraries | Multiplexing enables cost-efficient processing; unique molecular identifiers reduce duplicates |
| Spatial Transcriptomics | Capture slides with barcoded oligos, permeabilization reagents | Maintain spatial context while capturing RNA | Optimization needed for plant tissues with rigid cell walls |
Workflow Validation and Quality Control
Rigorous validation is essential for generating reliable multi-omics data. MEANtools was validated using a paired transcriptomic-metabolomic dataset generated to reconstruct the falcarindiol biosynthetic pathway in tomato, where it correctly anticipated five out of seven steps of the characterized pathway [24] [25]. This demonstrates the potential for such approaches to generate testable hypotheses even with incomplete pathway capture.
For metabolomics, implementation of quality controls includes using internal standards, pooled quality control samples, and solvent blanks throughout the analysis [26]. For transcriptomics, RNA integrity number (RIN) measurements should exceed 8.0 for reliable results, with careful monitoring of potential batch effects that can confound integration with metabolomics data. In spatial transcriptomics, validation through in situ hybridization techniques such as RNAscope provides confirmation of expression patterns identified through sequencing-based approaches [27].
Data security represents an increasingly important consideration, particularly when handling human genomic data or proprietary biosynthetic pathways. Leading platforms now implement advanced encryption protocols, secure cloud storage solutions, and strict access controls based on the principle of least privilege [29]. These measures are essential for protecting sensitive genetic information while enabling collaborative research.
The field of multi-omics integration is rapidly evolving, with several emerging trends shaping its future application to biosynthetic pathway elucidation. Spatial multi-omics technologies that simultaneously capture transcriptomic and metabolomic data within tissue context represent a promising frontier, though their application to plants requires overcoming significant technical hurdles [27]. Artificial intelligence continues to transform data analysis, with specialized models trained specifically on genomic data achieving increasingly precise interpretation of complex datasets [29] [30]. Additionally, efforts to democratize access to these technologies through cloud-based platforms and reduced sequencing costs are making multi-omics approaches available to smaller laboratories and institutions in underserved regions [29].
The integration of genomics, transcriptomics, and metabolomics within unified workflows has fundamentally transformed biosynthetic pathway discovery research. By moving beyond traditional targeted approaches that require prior knowledge, unsupervised computational methods like MEANtools can generate novel hypotheses about pathway components and connections [24] [25]. This paradigm shift is accelerating the characterization of specialized metabolic pathways in plants and microbes, with significant implications for drug development, agricultural improvement, and synthetic biology. As these technologies continue to mature and integrate, they promise to illuminate the vast remaining "dark matter" of metabolism, unlocking new biological insights and applications.
Leveraging Differential Gene Expression Analysis to Identify Candidate Enzymes
Elucidating biosynthetic pathways for valuable natural products constitutes a fundamental challenge in metabolic engineering and drug discovery research. While specialized metabolites, including many pharmaceuticals, often originate from plants and microbes, their biosynthetic pathways are frequently incomplete or unknown. This knowledge gap hinders efforts to engineer microbial hosts for sustainable production. Differential gene expression (DGE) analysis has emerged as a powerful methodological framework for identifying candidate enzymes within these pathways by systematically comparing transcriptional profiles under conditions of high and low metabolite production. This technical guide outlines integrated multi-omics approaches for linking gene expression patterns to biosynthetic function, providing researchers with robust protocols for candidate gene identification.
Theoretical Foundation of Differential Gene Expression Analysis
Differential gene expression analysis identifies genes that show statistically significant differences in expression levels between two or more biological conditions, such as different tissues, developmental stages, or treatments [31]. In biosynthetic pathway elucidation, this typically involves comparing systems with high versus low production of the target metabolite.
The statistical foundation of DGE analysis relies on measuring the probability that observed expression differences occurred by chance. Key parameters include:
- p-value: The probability of observing the data if no true difference exists (null hypothesis)
- p-adjust: Adjusted p-value correcting for multiple hypothesis testing to reduce false positives
- False Discovery Rate (FDR): The expected proportion of false positives among significant results
- log2 Fold Change (l2FC): The magnitude of expression difference between conditions [32] [31]
RNA-sequencing (RNA-Seq) has become the predominant method for DGE analysis, with computational tools like DESeq2 and EdgeR employing statistical models based on negative binomial distributions to account for technical and biological variability [32] [31]. These tools help control for false positives arising from multiple comparisons while maintaining sensitivity to detect true biological differences.
Integrated Multi-Omics Workflow for Enzyme Identification
Experimental Design Considerations
Effective identification of biosynthetic enzymes requires strategic experimental design that captures natural variation in metabolite production:
- Sample Selection: Collect tissues from multiple organs, developmental stages, and genetically distinct lines showing natural variation in target metabolite accumulation [33] [34]
- Replication: Include at least three biological replicates per condition to robustly estimate biological variance
- Metabolite Profiling: Conduct parallel metabolomic analysis (e.g., LC-MS) to quantify target compound levels across samples [33]
- Controls: Include appropriate control samples lacking the biosynthetic capability
Transcriptome Sequencing and Differential Analysis
The core transcriptomic workflow proceeds through the following stages:
Figure 1: Transcriptomic Analysis Workflow for Enzyme Discovery
Protocol: RNA Sequencing and Differential Expression Analysis
RNA Extraction and Quality Control
- Extract total RNA using kits such as Ultrature RNA Kit (Cowin Biotech) or equivalent [35]
- Assess RNA quality using Agilent 2100 Bioanalyzer or similar system
- Require RNA Integrity Number (RIN) >8.0 for library construction
Library Preparation and Sequencing
Bioinformatic Processing
- Filter raw reads to remove adapters, low-quality sequences, and reads with >10% N bases
- Assemble clean reads into transcripts using Trinity software [35]
- Cluster transcripts into unigenes using CD-HIT-EST
- Annotate unigenes against public databases (Nr, SwissProt, KEGG, COG, PFAM) using BLAST with E-value cutoff of 1×10⁻⁵ [35]
Differential Expression Analysis
- Quantify gene expression using RSEM to calculate FPKM (Fragments Per Kilobase of transcript per Million mapped reads) values [35]
- Identify differentially expressed genes (DEGs) using DESeq2 with threshold of FDR <0.05 and |log2FoldChange| >2 [35]
- Perform functional enrichment analysis (GO, KEGG) on DEG sets to identify overrepresented biosynthetic pathways
Integrative Analysis for Candidate Gene Prioritization
Once DEGs are identified, integration with complementary data types significantly enhances candidate gene prioritization:
Co-expression Network Analysis
- Construct weighted gene co-expression networks (WGCNA) to identify modules correlated with metabolite accumulation [34]
- Select hub genes within significant modules based on intramodular connectivity
Genome Mining and Retrosynthetic Approaches
- Utilize computational tools like BioNavi-NP to predict plausible biosynthetic routes [3]
- Match DEGs to predicted enzymatic steps in proposed pathways
- Cross-reference with known biosynthetic gene clusters in related organisms
Bulked Segregant Analysis (BSA-seq)
- For genetically diverse populations, perform BSA-seq on pools of individuals with extreme metabolite phenotypes [34]
- Identify genomic regions associated with trait variation
- Intersect positional candidate genes with DEGs from transcriptomic analysis
Case Studies in Biosynthetic Pathway Elucidation
Salicinoid Phenolic Glycosides in Populus tremula
Table 1: Candidate Genes for Salicinoid Biosynthesis Identified Through Integrated Analysis
| Gene Category | Candidate Genes Identified | Analysis Method | Functional Evidence |
|---|---|---|---|
| Acyltransferases | HXXXD-type acyltransferases (2 genes) | Differential analysis + co-expression | Correlation with cinnamoyl-containing SPGs |
| Glycosyltransferases | UDP-glucosyltransferase (1 gene) | Differential analysis + co-expression | Known role in glycoside formation |
| Sulfotransferases | SOT1 (previously validated) | Literature validation | Functional characterization in vivo |
Researchers identified candidate genes for salicinoid phenolic glycoside (SPG) biosynthesis in European aspen (Populus tremula) by integrating RNA-Seq and LC-MS data from multiple organs and genotypes producing contrasting SPG profiles [33]. The analysis combined gene and metabolite differential analyses with co-expression networks to pinpoint two HXXXD-type acyltransferase genes and one UDP-glucosyltransferase gene as candidates for enzymatic roles in attaching cinnamoyl moieties to SPG backbones [33].
Quercetin Biosynthesis in Euphorbia maculata
Table 2: Key Enzymes in Quercetin Biosynthetic Pathway Identified via Transcriptomics
| Enzyme | Abbreviation | Unigenes Identified | Role in Quercetin Pathway |
|---|---|---|---|
| Phenylalanine ammonia-lyase | PAL | 17 | Initial step from phenylalanine to cinnamic acid |
| Cinnamate 4-hydroxylase | C4H | 3 | Hydroxylation to 4-coumaric acid |
| 4-coumarate-CoA ligase | 4CL | 16 | Activation to 4-coumaroyl-CoA |
| Chalcone synthase | CHS | 5 | Condensation with malonyl-CoA to form chalcone |
| Chalcone isomerase | CHI | 4 | Isomerization to flavanone |
| Flavanone 3-hydroxylase | F3H | 1 | Hydroxylation to dihydroflavonol |
| Flavonoid 3′-hydroxylase | F3′H | 4 | Hydroxylation to quercetin precursor |
| Flavonol synthase | FLS | 9 | Final step to quercetin |
Transcriptome analysis of Euphorbia maculata across different tissues and developmental stages revealed 42 key DEGs associated with quercetin biosynthesis [35]. Researchers identified structural genes encoding all eight enzymes in the phenylpropanoid-flavonoid pathway leading to quercetin, with expression patterns correlating with tissue-specific quercetin accumulation patterns [35].
Tocopherol Biosynthesis in Brassica napus
In rapeseed (Brassica napus), researchers combined transcriptome analysis with BSA-seq to identify candidate genes regulating tocopherol (vitamin E) biosynthesis [34]. The study compared high- and low-vitamin E lines across seed developmental stages, identifying four key regulatory modules through WGCNA and seven hub genes involved in chlorophyll catabolism and vitamin E biosynthesis [34]. This integrated approach highlighted the connection between chlorophyll degradation and tocopherol synthesis, with five candidate genes (including BnA03g0107720) proposed as critical regulators.
Table 3: Key Databases and Tools for Biosynthetic Enzyme Discovery
| Resource Category | Database/Tool | Function | URL/Access |
|---|---|---|---|
| Sequence Databases | UniProt | Protein sequence and functional information | https://www.uniprot.org/ |
| PDB | 3D protein structures | https://www.rcsb.org/ | |
| Pathway Databases | KEGG | Metabolic pathways and enzyme annotations | https://www.kegg.jp/ |
| MetaCyc | Metabolic pathways and enzymes | https://metacyc.org/ | |
| Reactome | Biological pathways | https://reactome.org/ | |
| Compound Databases | PubChem | Chemical structures and properties | https://pubchem.ncbi.nlm.nih.gov/ |
| ChEBI | Small molecular compounds | https://www.ebi.ac.uk/chebi/ | |
| Enzyme Databases | BRENDA | Comprehensive enzyme information | https://brenda-enzymes.org/ |
| AlphaFold DB | Predicted protein structures | https://alphafold.ebi.ac.uk/ | |
| Analysis Tools | DESeq2 | Differential expression analysis | Bioconductor package |
| BioNavi-NP | Retrobiosynthesis prediction | http://biopathnavi.qmclab.com/ | |
| Selenzyme | Enzyme reaction prediction | Web tool |
Pathway Mapping and Visualization
Figure 2: Quercetin Biosynthetic Pathway with Candidate Enzymes [35]
Differential gene expression analysis provides a powerful foundation for identifying candidate enzymes in biosynthetic pathways, particularly when integrated with metabolomic data, co-expression networks, and computational predictions. The case studies presented demonstrate how multi-omics approaches can successfully pinpoint genes encoding enzymes for specialized metabolite biosynthesis, enabling subsequent functional characterization and metabolic engineering. As computational tools like BioNavi-NP continue to advance—achieving 72.8% accuracy in recovering known building blocks—the integration of rule-free deep learning models with experimental transcriptomic data will further accelerate the elucidation of complex biosynthetic pathways [3]. This methodology framework provides researchers with a systematic approach to overcome one of the most significant challenges in natural product research and synthetic biology applications.
Artificial Intelligence and Machine Learning in Predictive Pathway Modeling
The elucidation of biosynthetic pathways represents a fundamental challenge in biological research, with profound implications for drug discovery, natural product development, and synthetic biology. Predictive pathway modeling has emerged as a transformative approach that leverages artificial intelligence (AI) and machine learning (ML) to decipher the complex enzymatic reactions and regulatory networks that govern the synthesis of biologically active compounds. This paradigm shift from traditional trial-and-error methods to data-driven prediction is accelerating our ability to harness nature's chemical diversity for therapeutic applications.
The pharmaceutical industry's growing investment in AI technologies underscores their strategic importance. By 2025, AI spending in the pharmaceutical industry is expected to reach $3 billion, with AI projected to generate between $350 billion and $410 billion annually for the sector [36]. This substantial investment reflects the recognition that AI-driven approaches can significantly compress drug development timelines – from the typical 10-15 years to potentially just 12-18 months for certain candidates – while reducing discovery costs by up to 40% [36] [37].
At its core, predictive pathway modeling addresses the fundamental challenge that complete biosynthetic pathways, including all intermediates, are not established for most of the hundreds of thousands of known natural products [3]. While plants produce an enormous reservoir of medicinal compounds, the complex biosynthetic pathways of many plant-derived compounds remain only partially understood, hindering their full potential in therapeutic applications [17]. AI and ML technologies are now overcoming these limitations by integrating multi-omics data, identifying patterns beyond human perception, and generating testable hypotheses about biosynthetic routes.
Computational Frameworks and Architectures
Core Machine Learning Approaches
Predictive pathway modeling employs a diverse array of ML architectures, each optimized for specific aspects of pathway elucidation. The selection of appropriate algorithms depends on data availability, problem complexity, and desired output.
Deep learning models have demonstrated remarkable performance in bio-retrosynthesis prediction. The BioNavi-NP toolkit utilizes transformer neural networks trained on both general organic and biosynthetic reactions through end-to-end neural networks [3]. This system employs an AND-OR tree-based planning algorithm for iterative multi-step bio-retrosynthetic route planning, achieving a 72.8% accuracy in recovering reported building blocks from test compounds – 1.7 times more accurate than conventional rule-based approaches [3]. The model's performance significantly improves when trained on both biochemical data (31,710 reactions) and natural product-like organic reactions (60,000 reactions), with top-10 accuracy increasing from 27.8% to 60.6% [3].
Neural networks also power tools like NPBdetect, which predicts biological activity from biosynthetic gene clusters (BGCs) [38]. This approach addresses class imbalance issues through class weighting techniques and incorporates latest genome mining tools with novel sequence-based descriptors to enhance prediction accuracy for multiple bioactivities.
Explainable AI (XAI) methodologies have gained prominence as critical components for building trust in AI-driven pathway predictions. By 2025, advancements in XAI have largely solved the "black box" problem that once plagued AI systems, with 75% of organizations using AI and ML having implemented XAI to improve model interpretability [39]. These systems can now explain their predictions in business-friendly terms, bridging the gap between technical complexity and executive understanding, which has led to increased trust and adoption among stakeholders.
Data Processing Infrastructure
The architecture supporting predictive AI solutions requires robust data infrastructure spanning multiple specialized stages:
Table: Predictive AI Architecture Components
| Architecture Stage | Key Functions | Tools & Technologies |
|---|---|---|
| Data Analysis & Preparation | Data gathering, cleaning, quality enhancement, handling missing values, outlier removal | Big data platforms, data governance frameworks |
| Model Training & Validation | Algorithm selection, parameter adjustment, performance evaluation, ensemble methods | Transformer neural networks, random forests, neural networks |
| Deployment & Integration | Model serving, API integration, workflow incorporation, feedback loops | MLOps tools, real-time scoring services, batch processing |
| Scalable Infrastructure | High-speed data access, distributed processing, low-latency response | In-memory databases, key-value stores, NoSQL databases |
The data analysis and preparation phase is particularly crucial, as ML algorithms require large, high-quality datasets for optimal performance [40]. Data engineers improve data quality by handling missing values, removing outliers, and resolving inconsistencies to ensure reliable training data. For pathway prediction, this often involves aggregating years' worth of multi-omics information across diverse biological systems.
Model training and validation employs various ML algorithms – from linear regression for straightforward trends to decision trees for complex pattern recognition and neural networks for highly complex, non-linear relationships [40]. The choice of algorithms depends on the problem characteristics and available data. During training, models iteratively adjust internal parameters to learn relationships between input factors and outcomes until predictions closely match known results in training data.
Deployment patterns vary based on application requirements. Batch prediction runs models on a schedule to process large datasets, while real-time prediction serves models behind APIs integrated into applications for immediate decision-making [40]. In both cases, technical teams must integrate model outputs into business workflows, whether through applications showing recommendations to users, dashboard alerts for managers, or automated actions such as scheduling experiments.
Experimental Protocols and Methodologies
Integrated Multi-Omics Pathway Analysis
The elucidation of complex biosynthetic pathways requires the integration of multiple experimental approaches that generate vast datasets for computational analysis. The following workflow illustrates the standard protocol for AI-driven pathway discovery:
Multi-omics data generation forms the foundation of modern pathway elucidation. Researchers collect relevant plant tissues, organs, or cells to extract RNA and DNA materials for constructing transcriptomic and genomic profiles [21]. Simultaneously, untargeted or targeted metabolomics analyses are performed on the same tissues to establish transcriptome-metabolome correlation networks [21]. The enormous volume and intricacy of genomics, transcriptomics, and metabolomics data require robust tools for data management (acquisition, processing, and storage) and mining (data visualization, co-regulation, and correlation) [21].
Computational analysis begins with robust bioinformatic processing to identify candidate genes/enzymes or predict biosynthetic pathways. Candidate genes for any single step can be selected using various features:
- Homology-based screening: BLAST search against enzymes catalyzing predicted reactions
- Co-expression analysis: Expression profiles in relation to previously elucidated pathway genes
- Genomic proximity: Synteny analysis and cluster identification [21]
Experimental validation confirms computational predictions through multiple approaches:
- Virus-induced gene silencing (VIGS): Used to confirm gene function in planta, as demonstrated in safflower where silencing CtCGT and CtF6H reduced HSYA content by 29.6% and 30.8% respectively [23]
- Heterologous expression: Candidate genes cloned into expression vectors and transformed into heterologous hosts (E. coli, S. cerevisiae, N. benthamiana)
- In vitro enzyme assays: Biochemical characterization of purified recombinant proteins [21]
The Agrobacterium-mediated transient expression in N. benthamiana has particularly accelerated functional characterization of plant biosynthetic enzymes, allowing rapid co-expression of multiple metabolic genes with significantly less engineering effort compared to E. coli or yeast systems [21].
AI-Enabled Retrobiosynthesis Planning
For natural products with unknown pathways, AI-driven retrobiosynthesis provides a systematic approach to pathway design:
Single-step retrobiosynthesis employs deep learning models to generate candidate precursors for target natural products. The BioNavi-NP system uses transformer neural networks trained on biochemical reactions (33,710 unique pairs of precursors and metabolites) and augmented with 62,370 organic reactions similar to biochemical reactions [3]. This transfer learning approach significantly improves model robustness by learning general patterns and avoiding over-fitting. The ensemble of four optimal transformer models achieves top-1 and top-10 accuracies of 21.7% and 60.6% respectively – 1.7 times more accurate than rule-based approaches [3].
Multi-step pathway planning leverages the AND-OR tree-based search algorithm to solve the combinatorial number of options caused by branched synthetic pathways [3]. This approach efficiently samples plausible biosynthetic pathways through iterative multi-step bio-retrosynthetic routes, successfully identifying pathways for 90.2% of test compounds [3]. The system further evaluates plausible enzymes for each biosynthetic step using enzyme prediction tools like Selenzyme and E-zyme 2 [3].
Pathway reconstruction utilizes the identified building blocks and enzymatic steps to design reconstructible pathways in heterologous hosts. The vast chemical space of natural products is reachable from just four well-known biosynthetic pathways using essential building blocks: (1) acetic acid/malonic acid pathway for fatty acids, phenols, and polyketides; (2) mevalonic acid/methylerythritol phosphate pathway for terpenoids and steroids; (3) cinnamic acid/shikimic acid pathway for flavonoids, phenylpropanoids, lignans, and coumarins; and (4) amino acids pathway for alkaloids and peptides [3].
Key Research Reagents and Computational Tools
Essential Research Reagents
Table: Key Research Reagents for Pathway Elucidation
| Reagent/Category | Specific Examples | Research Application |
|---|---|---|
| Heterologous Host Systems | Escherichia coli, Saccharomyces cerevisiae, Nicotiana benthamiana | Functional characterization of candidate biosynthetic enzymes through heterologous expression |
| Cloning & Expression Systems | Expression vectors, Agrobacterium-mediated transformation | Gene cloning and recombinant protein production for enzyme activity assays |
| Analytical Standards | Reference compounds for metabolomics (naringenin, carthamidin, HSYA) | Metabolite identification and quantification through LC-MS comparison |
| Gene Silencing Systems | Virus-induced gene silencing (VIGS), RNA interference (RNAi) | Functional validation of candidate genes in planta through targeted silencing |
| Enzyme Assay Components | Purified enzymes, substrates, cofactors (NADPH, UDP-glucose) | In vitro biochemical characterization of enzyme function and kinetics |
Specialized Computational Tools
Table: Computational Tools for Predictive Pathway Modeling
| Tool Name | Application | Key Features | Performance Metrics |
|---|---|---|---|
| BioNavi-NP | Bio-retrosynthesis prediction | Transformer neural networks, AND-OR tree search, ensemble methods | 72.8% building block recovery (1.7x rule-based), 90.2% pathway identification success [3] |
| NPBdetect | Bioactivity prediction from BGCs | Neural networks, class imbalance handling, sequence-based descriptors | Multiple bioactivity detection with high confidence [38] |
| RetroPathRL | Rule-based retrobiosynthesis | Reaction rules, retrosynthetic planning | Benchmark for deep learning approaches [3] |
| AntiSMASH | BGC identification | Genome mining, cluster prediction | Latest version used for BGC characterization [38] |
| Selenzyme/E-zyme 2 | Enzyme prediction | Reaction rule application, genomic context analysis | Enzyme recommendation for predicted biosynthetic steps [3] |
Case Study: HSYA Biosynthesis Elucidation
The elucidation of hydroxysafflor yellow A (HSYA) biosynthesis demonstrates the powerful integration of predictive modeling with experimental validation. HSYA is a clinical investigational new drug for treating acute ischemic stroke, with a unique quinochalcone di-C-glycoside structure exclusively found in safflower (Carthamus tinctorius) flowers [23].
Researchers employed a comprehensive approach combining transcriptomics, co-expression analysis, and functional characterization to identify the complete HSYA pathway. The investigation began with tissue-specific metabolite profiling using LC-MS, which confirmed HSYA's exclusive presence in flowers [23]. This spatial distribution provided critical clues about pathway activity.
Bioinformatics analysis identified candidate genes through:
- Collection of transcriptome data from budding flowers, blooming flowers, calyx, and leaf tissues
- Identification of 306 UGT and 616 P450 transcripts using Pfam database features
- Co-expression analysis using chalcone synthase (CtCHS2) as bait, revealing 22 UGT and 24 P450 genes with strong correlation (r ≥ 0.8, Pearson coefficient) [23]
Functional characterization confirmed four key enzymes in the HSYA pathway:
- CtF6H: Flavanone 6-hydroxylase (cytochrome P450) that catalyzes 6-hydroxylation of naringenin to produce carthamidin
- CtCHI1: Chalcone-flavanone isomerase responsible for isomerization between carthamidin and isocarthamidin
- CtCGT: Flavonoid di-C-glycosyltransferase that attaches glycosyl groups
- Ct2OGD1: 2-oxoglutarate-dependent dioxygenase that coordinates with CtCGT to convert carthamidin or isocarthamidin to HSYA [23]
Experimental validation employed multiple approaches:
- VIGS-mediated silencing of CtCGT and CtF6H reduced HSYA content by 29.6% and 30.8% respectively
- In vitro enzyme assays confirmed CtCGT's ability to accept phloretin and 2-hydroxynaringenin as substrates
- Heterologous expression in N. benthamiana enabled de novo HSYA biosynthesis
- Microsomal assays demonstrated CtF6H's 6-hydroxylation activity with maximum activity at 4°C [23]
This case study exemplifies how predictive modeling guides targeted experimentation, accelerating pathway elucidation from years to months while providing the foundation for green, efficient production of valuable medicinal natural products.
Emerging Trends and Future Perspectives
Predictive pathway modeling is evolving rapidly, with several transformative trends shaping its future development and application across pharmaceutical and biotechnology sectors.
Multimodal AI models represent a significant advancement, with capabilities to process and analyze diverse data types simultaneously – including text, images, video, audio, and sensor data [39]. This enables more holistic predictions and comprehensive understanding of biological systems. The global multimodal AI market is expected to grow from $1.4 billion in 2020 to $12.8 billion by 2025, at a Compound Annual Growth Rate (CAGR) of 33.4% [39]. In retail applications, companies like Amazon and Walmart already use multimodal AI to analyze customer behavior and preferences by combining social media data, customer reviews, and sales transactions [39]. Similar approaches are being adapted for biological pathway analysis, integrating genomic, transcriptomic, proteomic, and metabolomic datasets.
Digital twin technology is emerging as a powerful approach for clinical trial optimization and biological system modeling. Companies like Unlearn create AI-driven models that predict how a patient's disease may progress over time, allowing pharmaceutical companies to design clinical trials with fewer participants while maintaining statistical power [41]. These digital twins simulate how a patient's condition might evolve without treatment, enabling researchers to compare real-world effects of experimental therapies against predicted outcomes [41]. This approach significantly reduces both the cost and duration of clinical trials – particularly valuable in therapeutic areas like Alzheimer's, where trial costs can exceed £300,000 per subject [41].
Generative AI advances are revolutionizing molecular design, with models like AlphaFold and Genie predicting protein structures with remarkable accuracy from amino acid sequences [36]. These capabilities are accelerating drug discovery by enabling more precise target identification and compound optimization. The pharmaceutical AI market continues substantial growth, expected to increase from $1.94 billion in 2025 to approximately $16.49 billion by 2034, accelerating at a CAGR of 27% from 2025 to 2034 [36].
FAIR data principles implementation is becoming critical for advancing predictive pathway modeling. Most publicly available datasets currently lack appropriate metadata, standardized formatting, or transparent access links [21]. The FAIR (Findability, Accessibility, Interoperability, and Reusability) principles are essential for making data sharing more efficient and ensuring original contributors receive proper citation and recognition when their datasets are reused [21]. This standardization facilitates reproducibility and ethical reuse while providing equal access to data-driven innovation – particularly important as AI tools increasingly depend on large, well-annotated datasets for training.
The convergence of these technologies suggests a future where predictive pathway modeling becomes increasingly accurate, efficient, and integrated across the drug development pipeline. As AI systems become more sophisticated and biological datasets continue expanding, we can anticipate unprecedented capabilities for deciphering nature's chemical complexity and harnessing it for therapeutic advancement.
Elucidating the biosynthetic pathways of plant natural products (PNPs) is a fundamental pursuit in biomedical and agricultural research, yet a significant bottleneck persists in translating genetic and biochemical discoveries into scalable production. Heterologous reconstruction—the process of assembling and expressing biosynthetic pathways in genetically tractable host organisms—has emerged as a transformative solution. This approach allows researchers to functionally validate predicted pathways, overcome the low-yield and recalcitrance issues inherent in native producers, and establish platforms for sustainable biomanufacturing [42] [43]. The choice of host system is critical, with the field largely divided between microbial platforms like Escherichia coli and Saccharomyces cerevisiae, and plant-based chassis, foremost among them Nicotiana benthamiana [42] [44]. This guide provides an in-depth technical comparison of these systems, detailing their respective advantages, methodologies, and applications within the iterative Design-Build-Test-Learn (DBTL) cycle that drives modern pathway elucidation and engineering [42].
Host System Comparison: Microbial vs. Plant-Based Chassis
The selection of an appropriate heterologous host is dictated by the complexity of the target molecule, the nature of the required enzymatic transformations, and the desired production scale. Microbial and plant-based systems offer complementary strengths and limitations.
Table 1: Comparative Analysis of Heterologous Production Platforms
| Feature | Microbial Systems (E. coli, Yeast) | N. benthamiana System |
|---|---|---|
| Genetic Tractability | High; well-established tools for rapid gene manipulation and screening [42] | High; amenable to both stable transformation and rapid transient expression [44] |
| Growth Cycle | Very fast (hours) [42] | Relatively fast (weeks); requires greenhouse/controlled environment [44] |
| Pathway Complexity | Suitable for pathways with soluble plant-derived enzymes; struggles with multi-P450 pathways and large protein complexes [42] | Excellent; native eukaryotic machinery supports complex pathways involving P450s, membrane-bound enzymes, and metabolons [42] [44] |
| Post-Translational Modifications | Limited in prokaryotes; yeast performs some eukaryotic modifications [42] | Full suite of eukaryotic PTMs; proper folding and compartmentalization [42] [43] |
| Toxicity & Compartmentalization | Limited capacity; product toxicity can impair cell growth and yield [42] | High inherent capacity; natural organelles (e.g., plastids, vacuoles) sequester toxic intermediates/products [42] [43] |
| Scalability | Excellent for industrial fermentation; established scale-up protocols [42] | Scalable biomass production; transient expression scales with agroinfiltration capacity [44] |
| Key Applications | Terpenoid precursors, simple alkaloids, pathway prototyping [42] | Complex terpenoids (e.g., saponins), flavonoids (e.g., diosmin), alkaloid intermediates, recombinant proteins [42] [44] |
Microbial Systems:E. coliandS. cerevisiae
Microbial chassis are prized for their rapid growth and well-characterized genetics. Early successes in synthetic biology were often achieved in these hosts, such as the production of terpenoid precursors in E. coli by engineering the mevalonate pathway [42] [43]. They are ideal for the initial screening of enzyme combinations and reconstructing core pathways. However, their limitations become apparent with complex plant metabolites. They often lack the necessary cellular environment—such as specific cytochrome P450 systems, subcellular compartments, or prenylation machinery—for the biosynthesis of many pharmaceuticals, leading to issues with enzyme insolubility, incorrect folding, or an inability to perform final structural elaborations [42] [43]. Furthermore, microbial hosts can suffer from metabolic burden and toxicity when accumulating non-native compounds [42].
The Plant Chassis:Nicotiana benthamiana
N. benthamiana has become a premier plant-based platform for pathway reconstruction. This allotetraploid plant in the Solanaceae family is not a natural producer of many high-value pharmaceuticals, making it a "blank slate" for engineering. Its major advantages include [44]:
- Rapid, High-Yield Transient Expression: The leaves are highly amenable to Agrobacterium tumefaciens-mediated transient transformation (agroinfiltration), allowing for rapid expression of multiple genes within days without the need for stable transformation [42] [44].
- Eukaryotic Protein Machinery: It possesses an extensive endoplasmic reticulum (ER) system, active plastids, and other organelles that are essential for the correct processing, folding, and activity of complex plant enzymes, particularly cytochrome P450s and glycosyltransferases [44].
- High Metabolic Flux and Compartmentalization: The plant's native metabolism provides ample precursors, and its cellular compartments can be harnessed to isolate and stabilize metabolic intermediates [42].
This system has been successfully used to reconstruct lengthy and complex pathways, such as the production of the vaccine adjuvant QS-7 saponin, which required the coordinated expression of 19 pathway genes, including multiple P450s and glycosyltransferases, yielding 7.9 µg/g Dry Weight [42] [43].
Computational and Omics Tools for Pathway Design
The first step in heterologous reconstruction is the confident prediction of a complete biosynthetic pathway. This process has been revolutionized by the integration of multi-omics data and computational biology.
Diagram 1: Bioinformatics workflow for biosynthetic pathway elucidation, from a target molecule to a list of candidate genes for heterologous testing.
Leveraging Biological Big Data
The effectiveness of computational design rests on the quality of underlying biological databases [16]. Key resources include:
Table 2: Essential Databases for Biosynthetic Pathway Design
| Data Category | Database Examples | Primary Function |
|---|---|---|
| Compounds | PubChem, ChEBI, NPAtlas, LOTUS [16] | Provides chemical structures, properties, and bioactivities of known metabolites. |
| Reactions/Pathways | KEGG, MetaCyc, Reactome [16] | Curates known enzymatic reactions and metabolic pathways across organisms. |
| Enzymes | UniProt, BRENDA, PDB, AlphaFold DB [16] | Offers detailed information on enzyme functions, kinetics, and 3D structures. |
Integrated bioinformatics pipelines use these databases to perform co-expression analysis (identifying genes whose expression patterns correlate with metabolite abundance), homology-based screening (finding enzymes similar to those in known pathways), and genomic cluster identification (locating physically linked biosynthetic genes) [17] [21]. For example, the elucidation of the strychnine and camptothecin pathways relied heavily on co-expression analysis of transcriptomic and metabolomic data [21].
Experimental Protocols for Heterologous Reconstruction
Pathway Assembly and Transformation inN. benthamiana
The reconstruction of a biosynthetic pathway in N. benthamiana typically follows a well-established workflow centered on agroinfiltration.
Diagram 2: The transient expression workflow in N. benthamiana for rapid pathway testing.
Detailed Methodology:
Vector Construction (The "Build" Phase): Codon-optimized genes for plant expression are cloned into a binary vector under the control of a strong constitutive plant promoter (e.g., Cauliflower Mosaic Virus 35S promoter). For multi-gene pathways, this may involve assembling individual constructs or using advanced gene-stacking techniques to create polycistronic vectors [44].
Agrobacterium Transformation and Culture Preparation:
- The expression vectors are introduced into a suitable Agrobacterium tumefaciens strain (e.g., GV3101).
- A single colony is used to inoculate a small liquid culture with appropriate antibiotics. After ~24 hours, this culture is used to inoculate a larger induction medium (e.g., Luria-Bertani or YEP) supplemented with antibiotics, 10 mM 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 5.6), and 200 µM acetosyringone.
- Cultures are grown to an optical density (OD600) of ~0.5-1.0. The cells are then pelleted by centrifugation and resuspended in an infiltration buffer (10 mM MgCl2, 10 mM MES, pH 5.6, and 200 µM acetosyringone) to a final OD600 of typically 0.1-0.5 per bacterial strain. For multi-gene pathways, equal volumes of individual agrocultures are mixed to create the final infiltration cocktail [44].
Leaf Infiltration (Agroinfiltration):
- The abaxial side of leaves from 4-6 week-old N. benthamiana plants is used for infiltration.
- The bacterial suspension is drawn into a needleless syringe, which is pressed against the leaf surface while gently supporting the other side. Pressure is applied to infiltrate the suspension, causing a water-soaked appearance in the infiltrated area.
- Multiple spots or entire leaves can be infiltrated for larger-scale experiments [44].
Incubation and Harvest (The "Test" Phase):
- Infiltrated plants are maintained under standard greenhouse or growth chamber conditions for 4-7 days.
- During this time, the Agrobacteria transfer the T-DNA containing the pathway genes to the plant cells, leading to transient expression and metabolite production.
- Leaf discs or entire infiltrated leaves are harvested, often flash-frozen in liquid nitrogen, and stored at -80°C until analysis [42] [44].
Metabolite Analysis:
- Metabolites are extracted from ground leaf tissue using solvents like methanol or ethyl acetate.
- Analysis is primarily performed via Liquid Chromatography-Mass Spectrometry (LC-MS) or Gas Chromatography-Mass Spectrometry (GC-MS) to detect, identify, and quantify the target compound and potential intermediates [42] [45].
- This quantitative data feeds into the "Learn" phase, where computational models are refined to guide the next cycle of engineering, such as optimizing the ratio of pathway enzymes or boosting precursor supply [42].
Optimizing Pathway Performance inN. benthamiana
Successful reconstruction often requires more than simple gene expression. Key optimization strategies include:
- Boosting Precursor Supply: Engineering or overexpressing rate-limiting enzymes in endogenous pathways (e.g., the methylerythritol phosphate (MEP) pathway for terpenoids) to increase flux toward the target compound [44].
- Subcellular Targeting: Directing enzymes to specific organelles (e.g., chloroplasts, ER, or cytoplasm) using signal peptides can enhance catalytic efficiency, reduce toxicity, and bring enzymes closer to concentrated precursor pools [46] [44].
- Suppressing Competing Pathways: Using RNA interference (RNAi) or virus-induced gene silencing (VIGS) to downregulate endogenous genes that divert metabolic flux away from the desired product [44].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for Heterologous Reconstruction in N. benthamiana
| Reagent / Material | Function / Explanation | Example Use Case |
|---|---|---|
| Binary Vectors (e.g., pEAQ) | High-expression binary vectors for stable or transient expression in plants. | Cloning biosynthetic genes under strong constitutive promoters [44]. |
| Agrobacterium tumefaciens | A soil bacterium naturally capable of transferring DNA into plant cells; the workhorse for plant transformation. | Delivering expression constructs into N. benthamiana leaf cells via agroinfiltration [42] [44]. |
| Acetosyringone | A phenolic compound that induces the Agrobacterium Virulence (Vir) genes, essential for T-DNA transfer. | Added to Agrobacterium cultures and infiltration buffers to maximize transformation efficiency [44]. |
| Infiltration Buffer (MgCl₂, MES) | A buffered solution that maintains Agrobacterium viability and facilitates infiltration into the leaf apoplast. | The medium for resuspending and diluting Agrobacterium cultures immediately before infiltration [44]. |
| Liquid Chromatography-Mass Spectrometry (LC-MS) | The core analytical platform for separating, detecting, and quantifying metabolites and pathway intermediates. | Confirming the production of diosmin or other target compounds in leaf extracts [42] [45]. |
Heterologous reconstruction in model systems like microbes and N. benthamiana is the critical bridge between pathway elucidation and practical application. While microbial systems offer speed for prototyping and producing simpler molecules, N. benthamiana stands out as a powerful and versatile eukaryotic chassis capable of hosting the biosynthesis of the most complex plant-derived pharmaceuticals. The continued integration of advanced computational tools, multi-omics data, and refined experimental protocols for these hosts will undoubtedly accelerate the discovery and sustainable production of valuable natural products for drug development and beyond.
Overcoming Production Bottlenecks: From Pathway Engineering to Cellular Longevity
Addressing Metabolic Burden and Toxic Intermediate Accumulation
In the pursuit of engineering robust microbial cell factories for the production of valuable natural products and chemicals, metabolic engineers face two significant challenges: metabolic burden and toxic intermediate accumulation. Metabolic burden refers to the cellular stress and growth impairment resulting from the diversion of resources toward heterologous pathway expression and operation [47]. This burden often manifests as reduced host fitness, decreased product titers, and process instability. Similarly, the accumulation of toxic intermediates—whether from native metabolism or introduced pathways—can inhibit cell growth and sabotage production objectives [48]. Within the broader context of biosynthetic pathway elucidation and discovery research, addressing these challenges is paramount for transforming predictive biosynthetic models [3] [49] into industrially viable bioprocesses.
Core Concepts and Definitions
Metabolic burden arises from the energetic and biosynthetic demands imposed by heterologous pathway expression. Key sources include:
- Resource Competition: Heterologous enzymes compete with host proteins for finite cellular resources, including ATP, amino acids, and cofactors [47].
- Cellular Stress: Protein overexpression can trigger stress responses, misfolding, and inclusion body formation.
- Reduced Fitness: Burdened cells often exhibit slowed growth, elongated division times, and increased susceptibility to environmental fluctuations.
This burden is particularly pronounced in complex biosynthetic pathways, such as those for polyketides and nonribosomal peptides, where large, multi-domain enzymes (PKS/NRPS) must be expressed and functionally coordinated [49].
Toxic Intermediate Accumulation
Toxic intermediates can originate from:
- Unbalanced Pathway Flux: When the rate of intermediate production exceeds its consumption, leading to pooling.
- Chemical Reactivity: Some pathway intermediates are inherently reactive, damaging cellular components.
- Membrane Disruption: Hydrophobic or detergent-like compounds can compromise membrane integrity.
The recent development of tools like BioNavi-NP, which predicts biosynthetic pathways for natural products using deep learning, allows researchers to anticipate potential metabolic bottlenecks and toxicity issues in silico before experimental implementation [3].
Quantitative Assessment of Metabolic Challenges
Table 1: Representative Metabolic Engineering Cases Addressing Burden and Toxicity
| Target Product | Host Organism | Key Challenge | Engineering Strategy | Outcome | Reference |
|---|---|---|---|---|---|
| 3-Hydroxypropionic Acid | C. glutamicum | Metabolic Burden | Substrate Engineering & Genome Editing | 62.6 g/L, 0.51 g/g glucose | [48] |
| Lysine | C. glutamicum | Pathway Bottlenecks | Cofactor & Transporter Engineering | 223.4 g/L, 0.68 g/g glucose | [48] |
| Succinic Acid | E. coli | Toxic intermediate accumulation? | Modular Pathway Engineering & High-Throughput Genome Editing | 153.36 g/L, 2.13 g/L/h | [48] |
| Malonic Acid | Y. lipolytica | General Optimization | Modular Pathway, Genome & Substrate Engineering | 63.6 g/L, 0.41 g/L/h | [48] |
Table 2: Analytical Techniques for Monitoring Burden and Toxicity
| Technique | Measured Parameter | Application in Burden/Toxicity Assessment |
|---|---|---|
| MetaboAnalyst [28] | Metabolite concentrations, Pathway enrichment | Statistical and multivariate analysis of metabolomics data to identify accumulated intermediates and pathway dysregulation. |
| Flux Balance Analysis | Metabolic Flux | Constraint-based modeling to predict flux redistribution and identify ATP/redox imbalances indicative of burden. |
| RNA-Seq | Transcriptome | Identification of stress response signatures and dysregulated native genes. |
| Proteomics | Protein abundance | Quantification of heterologous enzyme expression and host proteome reallocation. |
Methodologies and Experimental Protocols
Dynamic Pathway Control to Relieve Metabolic Burden
Principle: Decouple cell growth from product synthesis using inducible systems or dynamic switches, thereby minimizing burden during rapid growth phases [47].
Protocol:
- Identify a Quorum-Sensing (QS) or Metabolic Sensor: Select a sensor that responds to a key intermediate or population density.
- Construct a Dynamic Circuit: Fuse the sensor's promoter to a repressor or activator protein that controls the heterologous pathway.
- Implement and Test:
- Introduce the circuit into the production host.
- In a bioreactor, monitor cell density (OD600), nutrient levels, and product titer.
- Validate sensor activation and pathway induction at the desired growth phase via qPCR and product concentration measurements.
- Optimize: Fine-tune the circuit's response threshold by modulating promoter strength or protein expression levels to maximize product yield.
Enzyme Engineering to Prevent Toxic Intermediate Accumulation
Principle: Enhance the kinetics or specificity of a rate-limiting enzyme to prevent the pooling of its toxic substrate.
Protocol:
- Pinpoint the Bottleneck Enzyme: Use metabolomics (e.g., via MetaboAnalyst [28]) to identify the accumulated intermediate. The enzyme preceding this intermediate is a candidate.
- Generate Enzyme Variants: Employ directed evolution or structure-guided rational design to create a library of enzyme variants.
- High-Throughput Screening:
- Express variants in a microbial host sensitive to the toxic intermediate.
- Use a fluorescence-based reporter or growth assay to select clones that show improved viability, indicating reduced intermediate accumulation.
- Validate: Introduce the top-performing variant into the full production pathway and measure the reduction in intermediate levels and the increase in final product titer.
Consortium Engineering for Spatial Segregation
Principle: Distribute a metabolically demanding pathway across multiple engineered microbial strains to isolate and mitigate burden and toxicity [47].
Protocol:
- Pathway Partitioning: Split the target biosynthetic pathway into two or more modules. Strategically place a toxic reaction in a dedicated "detoxification" strain.
- Strain Development: Engineer separate host strains, each containing a distinct pathway module and necessary genetic controls.
- Co-culture Optimization:
- Inoculate strains at a predetermined ratio in a bioreactor.
- Monitor strain population dynamics via flow cytometry (using fluorescent markers) and product formation.
- Adjust culture conditions (e.g., feeding strategy) to maintain a stable, productive consortium.
- Characterize: Measure the final product yield and stability of the co-culture compared to a single-strain approach.
Visualization of Engineering Strategies and Workflows
Diagram 1: Strategy selection workflow for addressing metabolic challenges.
Diagram 2: Dynamic control protocol for metabolic burden mitigation.
The Scientist's Toolkit: Essential Research Reagents and Platforms
Table 3: Key Software and Experimental Tools
| Tool/Reagent Name | Type | Primary Function in Research | Relevance to Burden/Toxicity |
|---|---|---|---|
| BioNavi-NP [3] | Software Platform | Predicts biosynthetic pathways for natural products using deep learning. | Enables in silico pathway design and identification of potential problematic (toxic) intermediates before construction. |
| RAIChU [49] | Software Platform | Automates visualization of natural product biosynthetic pathways (PKS, NRPS, RiPPs). | Aids in conceptualizing complex multi-enzyme pathways where burden and intermediate channeling are critical. |
| MetaboAnalyst [28] | Web Analysis Platform | Statistical and functional analysis of metabolomics data. | Identifies and quantifies accumulated toxic intermediates; performs pathway analysis to pinpoint dysregulated metabolism. |
| Inducible Promoter Systems | Genetic Part | Allows external (e.g., aTc, IPTG) or internal (QS) control of gene expression. | Core component of dynamic control strategies to decouple growth and production, relieving burden. |
| Fluorescent Reporter Proteins | Research Reagent | Visual tags (e.g., GFP, mCherry) for gene expression or strain tracking. | Used to report on promoter activity, stress response, and to monitor population ratios in microbial consortia. |
| Genome-Scale Metabolic Models (GEMs) | Modeling Framework | Computational models of organism metabolism. | Predicts ATP/redox imbalances and flux redistribution resulting from heterologous pathway expression (burden). |
Successfully addressing metabolic burden and toxic intermediate accumulation requires a holistic and multi-faceted approach. As outlined in this guide, strategies range from hierarchical metabolic engineering [48] and dynamic control [47] to the innovative use of predictive software like BioNavi-NP [3] and analytical platforms like MetaboAnalyst [28]. The integration of computational prediction, careful pathway design, and sophisticated genetic control enables researchers to navigate the complexities of biosynthetic pathway elucidation. By systematically applying these principles and tools, scientists can transform microbial hosts into efficient and robust cell factories, accelerating the discovery and sustainable production of high-value natural products and pharmaceuticals.
The elucidation and engineering of biosynthetic pathways represent a cornerstone of modern biotechnology, enabling the sustainable production of high-value natural products for pharmaceutical and industrial applications. However, traditional metabolic engineering approaches often encounter persistent roadblocks, including cellular toxicity from pathway intermediates, loss of flux to competing reactions, and inadequate product sequestration [50]. Spatial engineering has emerged as a transformative paradigm to address these challenges by deliberately organizing biochemical processes within cellular space. This approach harnesses and engineers the innate compartmentalization of eukaryotic cells and applies similar organizational principles to microbial hosts, creating optimized environments for biosynthetic pathways while mitigating cytotoxicity [51] [52]. For researchers engaged in pathway discovery and elucidation, understanding and applying spatial engineering strategies is crucial for translating identified pathways into efficient production systems. This technical guide examines current compartmentalization and transporter engineering methodologies, providing a framework for their implementation within biosynthetic pathway research and development.
Subcellular Compartment Engineering in Yeast
As a premier eukaryotic host for heterologous biosynthesis, Saccharomyces cerevisiae offers a well-characterized intracellular architecture that can be repurposed for metabolic engineering. Compartmentalization inherently protects the intracellular environment by sequestering toxic intermediates and metabolites within confined spaces, while simultaneously enhancing catalytic efficiency through substrate channeling and reduced cross-talk [51]. The table below summarizes the key organelles targeted for engineering and their respective advantages.
Table 1: Target Organelles for Compartment Engineering in Yeast
| Organelle | Native Physiological Functions | Advantages for Engineering | Example Products |
|---|---|---|---|
| Endoplasmic Reticulum (ER) | Protein synthesis, folding, & secretion; lipid synthesis; calcium storage [51]. | Extensive membrane surface; native location of cytochrome P450 enzymes; can be massively expanded [51] [52]. | Triterpenoids (e.g., β-amyrin) [52], Ginsenosides [51]. |
| Lipid Droplets (LDs) | Storage of neutral lipids (TAGs, SEs) [52]. | Natural sink for hydrophobic compounds; high storage capacity; surface可用 for enzyme anchoring [51] [52]. | Lycopene [52], α-Amyrin [52], Ginsenoside Compound K [52]. |
| Peroxisomes | Fatty acid β-oxidation; housing of specific oxidative reactions [51]. | Confined environment with selective membrane; can concentrate substrates and enzymes [51]. | Squalene [51], α-Farnesene [51]. |
| Mitochondria | TCA cycle, oxidative phosphorylation, apoptosis regulation [51]. | High acetyl-CoA availability; distinct ATP and NADPH pools; separate environment from cytosolic regulation [51]. | Isobutanol [51], 2-Methyl-1-butanol [51], Squalene [51]. |
Organelle-Specific Engineering Strategies
ER Engineering: A primary strategy for enhancing the biosynthetic capacity of the ER involves membrane proliferation. This is achieved by disrupting the phosphatidic acid phosphatase-encoding PAH1 gene or overexpressing the transcription factor INO2. These manipulations lead to a dramatic expansion of the ER membrane, increasing its capacity for hosting biosynthetic enzymes, particularly membrane-bound cytochrome P450s. For example, a Δpah1 strain in S. cerevisiae showed an 8-fold and 16-fold increase in the accumulation of the triterpenoids β-amyrin and medicagenic-28-O-glucoside, respectively [52].
LD Engineering: Engineering LDs focuses on two aspects: increasing their storage capacity and co-localizing enzymes with their hydrophobic substrates. Overexpression of diacylglycerol acyltransferase (DGA1 or YlDGA2) leads to the formation of larger or more numerous LDs, thereby enhancing the intracellular storage volume for lipophilic compounds like lycopene and α-amyrin [52]. Furthermore, enzymes can be targeted to the LD surface using anchor proteins like PLN1. This strategy was successfully used to relocate protopanaxadiol synthase to LDs, resulting in a 4.4-fold increase in the production of ginsenoside Compound K compared to the native ER-localized enzyme [52].
Mitochondria and Peroxisomes Engineering: These organelles offer unique biochemical environments. Mitochondria are engineered to harness their abundant acetyl-CoA pool for synthesizing terpenoid precursors, effectively creating a parallel biosynthetic hub that bypasses cytosolic regulation [51]. Peroxisomes, with their semi-permeable membrane, are utilized to sequester specific pathways, such as those for squalene and α-farnesene synthesis, minimizing interference with cytosolic metabolism and reducing intermediate toxicity [51].
Table 2: Key Genetic Modifications for Organelle Engineering
| Engineering Strategy | Genetic Manipulation | Physiological Outcome | Impact on Product Titer |
|---|---|---|---|
| ER Expansion | Deletion of PAH1 [52]. | Proliferation of ER membranes. | 8-16x increase for triterpenoids [52]. |
| ER Expansion | Overexpression of INO2 [52]. | Proliferation of ER membranes. | 128x increase in squalene, 7x increase in ginsenoside [52]. |
| LD Size/Number Control | Overexpression of DGA1 [52]. | Increased number of smaller LDs. | 106x increase for α-amyrin in yeast [52]. |
| LD Size/Number Control | Overexpression of YlDGA2 in Yarrowia [52]. | Formation of larger LDs. | Improved lycopene storage [52]. |
| Enzyme Anchoring to LDs | Fusion with LD anchor protein (e.g., PLN1) [52]. | Co-localization of enzyme and substrate on LD surface. | 4.4x increase for Ginsenoside Compound K [52]. |
| Blocking Competing Pathways | Deletion of GUT2, POX1-6 in Yarrowia [52]. | Increased precursor pool (GUT2), blocked β-oxidation (POX). | Enhanced lycopene yield (16 mg/g CDW) [52]. |
The following workflow outlines the decision process for selecting and implementing a compartmentalization strategy, integrating the considerations of pathway chemistry and host engineering.
Diagram 1: Compartmentalization Strategy Workflow
Transporter Engineering for Efflux and Sequestration
Even with efficient internal biosynthesis, end-product cytotoxicity and inadequate secretion can limit titers. Transporter engineering addresses this by enhancing efflux into the culture medium or facilitating sequestration into intracellular vacuoles [52] [53].
In plants, specific transporters for flavonoids have been well-characterized, providing a blueprint for engineering microbial transport systems. ATP-binding cassette (ABC) transporters, particularly multidrug resistance-associated proteins (MRPs), use ATP hydrolysis to actively transport flavonoid glycosides into the vacuole [53]. Multidrug and toxic compound extrusion (MATE) transporters utilize proton gradients to efflux flavonoids, functioning as H⁺/flavonoid antiporters [53]. Additionally, glutathione S-transferase (GST)-dependent mechanisms, where GSTs act as ligandins binding to anthocyanins, facilitate their transport to the tonoplast [53]. A fourth mechanism involves vesicle-mediated trafficking, where flavonoids are transported via the endoplasmic reticulum and Golgi apparatus to the vacuole [53].
Heterologous expression of these plant-derived transporters in microbial hosts is an emerging strategy to alleviate product inhibition and toxicity. Engineering efflux systems is particularly critical for achieving high yields in continuous bioprocessing, as it simplifies product recovery and reduces feedback inhibition.
Experimental Protocols for Spatial Engineering
Protocol: Engineering ER Expansion for Enhanced Triterpenoid Production
This protocol details the process of expanding the endoplasmic reticulum in S. cerevisiae to improve the yield of triterpenoid compounds [52].
Strain Construction:
- Gene Deletion: Design a knockout cassette for the PAH1 gene (encodes phosphatidic acid phosphatase). Transform into your base production strain using standard homologous recombination methods.
- Alternative Approach: Design an overexpression cassette for the transcription factor INO2 under a strong constitutive promoter.
Validation of ER Expansion:
- Microscopy: Use fluorescence microscopy to confirm ER proliferation. Stain the ER with a marker such as ER-Tracker Red or express an ER-targeted GFP (e.g., HDEL-GFP).
Pathway Engineering:
- Express the triterpenoid biosynthetic pathway genes, ensuring key enzymes (e.g., cytochrome P450s) are equipped with native ER-targeting signals.
Fermentation and Analysis:
- Cultivate engineered and control strains in appropriate medium.
- Extract metabolites from cell pellets using organic solvents (e.g., ethyl acetate or chloroform/methanol).
- Analyze triterpenoid yield using LC-MS or GC-MS. Compare titers between the Δpah1 or INO2-overexpressing strain and the control.
Protocol: Anchoring Biosynthetic Enzymes to Lipid Droplets
This protocol describes the re-localization of enzymes to the surface of lipid droplets to enhance access to hydrophobic substrates [52].
Gene Fusion Design:
- Fuse the coding sequence of your target enzyme (e.g., protopanaxadiol synthase for ginsenoside production) to the N- or C-terminus of a lipid droplet anchor protein like PLN1 from yeast. Separate the genes with a flexible linker sequence (e.g., GGSGG).
Strain Transformation and Screening:
- Clone the fusion construct into an appropriate expression vector and transform it into your production strain.
- Screen for positive clones and verify protein expression.
Validation of Localization:
- Use fluorescence microscopy to confirm correct localization. Co-express the fusion protein (tagged with e.g., GFP) with a lipid droplet-specific stain (e.g., Nile Red).
Productivity Assessment:
- Cultivate the engineered strain and analyze product formation as described in Protocol 4.1. Compare the yield to strains where the enzyme is localized to the ER or cytosol.
The Scientist's Toolkit: Essential Research Reagents
The following table lists key reagents and tools required for implementing spatial engineering strategies.
Table 3: Research Reagent Solutions for Spatial Engineering
| Reagent/Tool | Function | Example Use Case |
|---|---|---|
| Organelle-Specific Fluorescent Dyes (e.g., ER-Tracker, Nile Red, MitoTracker) | Visualizing and validating organelle morphology and size under microscopy. | Confirming ER expansion after PAH1 deletion [52]. |
| Anchor Protein Sequences (e.g., PLN1 for LDs, TOM70 for mitochondria, PTS1 for peroxisomes) | Genetically fusing to enzymes to direct their subcellular localization. | Anchoring protopanaxadiol synthase to LDs for ginsenoside production [52]. |
| Vectors for Constitutive/Inducible Expression (e.g., pRS series, GAL promoters) | Controlling the expression level and timing of pathway genes and engineering constructs. | Fine-tuning the expression of INO2 to control ER size [52]. |
| Heterologous Transporters (e.g., plant ABC transporters like TT12, MATE transporters) | Cloning into microbial hosts to enhance product efflux or vacuolar sequestration. | Alleviating feedback inhibition and product toxicity in yeast [53]. |
| CRISPR-Cas9 Tools for Yeast | Performing precise gene knockouts (e.g., PAH1, GUT2) and integrations. | Rapidly engineering host strains with expanded organelles or deleted competing pathways [51] [52]. |
Spatial engineering transcends traditional pathway optimization by introducing intracellular organization as a fundamental design parameter. The strategic compartmentalization of pathways within organelles and the engineering of transport systems directly address the critical bottlenecks of toxicity, intermediate loss, and low catalytic efficiency. For scientists engaged in biosynthetic pathway elucidation, integrating these spatial considerations from the outset is no longer an advanced tactic but a core component of constructing robust cell factories. As pathway discovery efforts unveil increasingly complex natural product targets, the application of compartmentalization and transporter engineering will be indispensable for translating these genetic blueprints into commercially viable and sustainable biomanufacturing processes.
Within the broader context of biosynthetic pathway elucidation and discovery, the productivity of microbial factories is a cornerstone for the sustainable production of high-value natural products, such as pharmaceuticals, biofuels, and specialty chemicals [21] [17]. However, cellular aging—the gradual decline in cellular function and eventual loss of viability—poses a significant barrier to achieving high yields and economically viable bioprocesses [54]. In microbial populations, replicative aging manifests as a decline in the ability of mother cells to produce subsequent daughters, while senescence can be triggered by various metabolic and environmental stresses. This aging process leads to reduced metabolic activity, increased cell-to-cell heterogeneity, and the accumulation of damaged proteins and DNA, ultimately diminishing the overall titers, rates, and yields (TRY) of the target compound. As the field moves towards elucidating and reconstructing increasingly complex plant natural product pathways in microbial hosts like Escherichia coli and Saccharomyces cerevisiae [21] [3], the imperative to overcome the limitations imposed by cellular aging intensifies. This technical guide explores the mechanisms of cellular aging in industrial microbes and details the experimental methodologies for quantifying, analyzing, and engineering extended lifespan to create more robust and productive microbial cell factories.
Quantifying Aging in Microbial Bioprocesses
To systematically engineer for extended lifespan, it is first necessary to quantify the impact of aging on bioprocessing parameters. The following table summarizes key metrics and the analytical techniques used for their measurement.
Table 1: Key Quantitative Metrics for Assessing Microbial Aging in Bioprocesses
| Metric Category | Specific Parameter | Measurement Technique | Implication for Biosynthesis |
|---|---|---|---|
| Population Viability | Percentage of viable cells | Flow cytometry with live/dead staining (e.g., propidium iodide) | Directly correlates with maintained metabolic activity and production capacity [54]. |
| Replicative Lifespan (RLS) | Mean/Median number of daughter cells produced by a mother cell | Microscopic dissection of mother cells (yeast); Time-lapse microfluidics coupled with image analysis | Determines the long-term replicative capacity of the production host [54]. |
| Metabolic Activity | ATP levels, NAD+/NADH ratio | Luminescent assays, Enzymatic cycling assays | Reflects the energetic state of the cell, crucial for driving energetically expensive biosynthetic pathways [54]. |
| Oxidative Stress | Intracellular ROS levels | Flow cytometry with fluorescent probes (e.g., H2DCFDA) | High ROS causes damage to lipids, proteins, and DNA, impairing enzyme function and pathway flux [54]. |
| Senescence-Associated Secretory Phenotype (SASP) | Extracellular proteases, cytokines/inflammatory mediators | LC-MS/MS for SASP factor identification, Enzyme activity assays | Can create a pro-aging extracellular environment, negatively impacting the entire population [54]. |
| Pathway-Specific Output | Titer of target natural product (e.g., µg/L) | LC-MS/MS, HPLC | The ultimate measure of how aging impacts the productivity of the engineered biosynthetic pathway [21] [17]. |
Experimental Protocols for Lifespan Analysis and Engineering
This section provides detailed methodologies for core experiments in microbial aging research, from fundamental quantification to advanced pathway engineering.
Protocol: Microfluidic Analysis of Replicative Lifespan inS. cerevisiae
Objective: To precisely track the replicative lifespan of individual yeast mother cells in a controlled environment while expressing a heterologous biosynthetic pathway.
Strain Preparation:
- Engineer a production strain of S. cerevisiae with the desired biosynthetic pathway genes integrated into the genome.
- Introduce a fluorescent reporter (e.g., GFP) under a constitutive promoter to facilitate cell tracking and visualization.
Chip Loading and Cultivation:
- Use a commercial microfluidic device (e.g., CellASIC ONIX) designed for yeast replicative aging.
- Load a mid-log phase culture of the engineered strain into the device's input reservoir.
- Apply constant medium flow (e.g., SC medium with necessary carbon sources and pathway inducers) using a programmable perfusion system. The medium is maintained at 30°C.
Time-lapse Imaging and Data Acquisition:
- Place the device on an automated inverted fluorescence microscope equipped with an environmental chamber.
- Program the microscope to capture bright-field and fluorescence images of multiple trapping sites every 10-15 minutes for the duration of the lifespan (typically 3-5 days).
- Use image analysis software (e.g., MicrobeTracker, Outfi) to automatically identify and track mother cells and their budding events.
Data Analysis:
- The Replicative Lifespan (RLS) for each mother cell is defined as the total number of buds produced before irreversible cell cycle arrest.
- Plot survival curves (percentage of mother cells still dividing vs. replicative age) and calculate the mean and median RLS.
- Correlate the replicative age with the fluorescence intensity of a pathway intermediate or product reporter, if available.
Protocol: Engineering Aging-Resistant Hosts via Adaptive Laboratory Evolution (ALE)
Objective: To select for mutants with enhanced lifespan and sustained production under industrial-like stress conditions.
Evolution Setup:
- Base Strain: Use an engineered production strain as the ancestor.
- Selection Pressure: Implement a serial passaging regime in a bioreactor or multi-well plates with conditions that mimic industrial stress, such as:
- Periodic starvation pulses (e.g., glucose limitation).
- Mild, chronic oxidative stress (e.g., sub-lethal concentrations of menadione or H2O2).
- Elevated temperature (e.g., 35°C for a S. cerevisiae strain optimized for 30°C).
- Product or intermediate toxicity (e.g., adding a toxic pathway intermediate to the medium).
Evolution and Monitoring:
- Passage the culture repeatedly for hundreds of generations, always transferring the most rapidly growing cells.
- Regularly sample the population to monitor:
- Phenotype: Growth rate, viability, and product titer.
- Genotype: Whole-genome sequencing of pooled populations to identify accumulating mutations.
Isolation and Validation:
- After a significant increase in fitness is observed, isolate single clones from the evolved population.
- Re-test the isolated clones for improved replicative lifespan using the microfluidic protocol (3.1) and for sustained product titer in batch or fed-batch fermentations compared to the ancestor strain.
- Use whole-genome sequencing to identify the causal mutations conferring the extended lifespan phenotype.
Protocol: Integrating Multi-Omics to Decipher Aging-Metabolism Interactions
Objective: To identify key molecular drivers of aging in an engineered microbial factory and pinpoint pathway bottlenecks exacerbated by senescence.
Sample Collection:
- Culture the engineered production strain and collect samples at multiple, distinct physiological timepoints: early exponential phase (young), mid-stationary phase (middle-aged), and late stationary phase (aged).
- Use techniques like fluorescence-activated cell sorting (FACS) with age-associated markers (e.g., surface composition, size) to physically separate young and old subpopulations from the same culture for analysis.
Multi-Omics Profiling:
- Transcriptomics: Extract total RNA and perform RNA-Seq. Analyze differential gene expression between age groups, focusing on biosynthetic pathway genes, stress response regulons, and metabolic transporters.
- Metabolomics: Quench metabolism rapidly and perform intracellular metabolomics via LC-MS/MS or GC-MS. Quantify central carbon metabolites, energy carriers (ATP/ADP/AMP, NAD+/NADH), and intermediates/products of the engineered pathway.
- Proteomics: Extract proteins and analyze via LC-MS/MS. Identify changes in the abundance of pathway enzymes, chaperones, and oxidative damage repair proteins.
Data Integration and Pathway Analysis:
- Integrate the multi-omics datasets using bioinformatics tools and custom scripts.
- Map the changes onto metabolic networks (using platforms like PathVisio [55]) and regulatory pathways to build a comprehensive model of how aging impacts the engineered system.
- Identify key nodes where metabolic flux declines with age, and prioritize these as targets for engineering (e.g., by swapping in more stable enzyme isoforms or implementing dynamic regulatory control).
Visualizing the Experimental Workflow for Engineering Long-Lived Microbial Factories
The following diagram illustrates the integrated workflow from aging phenotype analysis to the creation of an engineered, long-lived production host.
The Scientist's Toolkit: Essential Reagents and Solutions
The following table catalogs key reagents, tools, and their applications for researching and engineering cellular aging in microbial systems.
Table 2: Research Reagent Solutions for Microbial Aging Studies
| Reagent / Tool | Function / Application | Example Use Case |
|---|---|---|
| Microfluidic Devices | High-throughput, single-cell analysis of replicative lifespan under constant environmental conditions. | Real-time tracking of mother cell divisions and correlation with biosynthetic output via fluorescent reporters [54]. |
| Live/Dead Stains (e.g., Propidium Iodide) | Discrimination between viable and non-viable cells in a population based on membrane integrity. | Flow cytometric quantification of culture viability over the course of a fermentation run. |
| ROS-Sensitive Fluorescent Probes (e.g., H2DCFDA) | Detection and quantification of intracellular reactive oxygen species (ROS). | Measuring oxidative stress burden in young vs. aged subpopulations sorted from a production culture. |
| Senolytic Compounds (e.g., Dasatinib + Quercetin) | Selective induction of apoptosis in senescent cells [54]. | Pulsing a fermentation culture with senolytics to clear aged, non-productive cells and rejuvenate the population. |
| PathVisio | Biological pathway creation, editing, and analysis [55]. | Visualizing and modeling the impact of aging on the flux through an engineered biosynthetic pathway by overlaying omics data. |
| BioNavi-NP | Deep learning-based prediction of biosynthetic pathways for natural products [3]. | Designing and optimizing the heterologous pathway itself to be less burdensome or to avoid the production of pro-aging toxic intermediates. |
| CRISPRi/a Systems | Targeted knockdown (interference) or activation (activation) of gene expression without genetic modification. | Tunably repressing aging-driver genes (e.g., TOR1) or activating longevity-associated genes (e.g., SIR2) in production strains. |
| LC-MS/MS | Highly sensitive and specific identification and quantification of metabolites, proteins, and SASP factors. | Profiling the intracellular metabolome of aged cells to identify pathway bottlenecks or detecting SASP factors in the culture supernatant [21] [54]. |
The systematic engineering of extended cellular lifespan is no longer a peripheral concern but a central strategy for maximizing the potential of microbial factories in the realm of natural product biosynthesis. By moving beyond traditional metrics like final titer to incorporate quantitative measures of cellular aging, and by employing integrated experimental-computational workflows, researchers can directly address the root causes of process instability and declining productivity. The methodologies detailed herein—from single-cell lifespan analysis and adaptive evolution to multi-omics integration and targeted genetic interventions—provide a robust toolkit for deconstructing the complex interplay between aging and metabolism. As AI-powered tools like BioNavi-NP continue to refine our ability to design optimal biosynthetic pathways [3] [56], and as our fundamental understanding of microbial senescence deepens [54], the deliberate engineering of longevity will become a standard, indispensable component in the development of next-generation, industrially resilient microbial cell factories.
Automated Platforms and Evolutionary Selection for High-Performance Strain Development
The transition to a sustainable bioeconomy and the acceleration of drug development are increasingly dependent on our ability to engineer microbial strains that efficiently produce valuable compounds. Traditional methods for strain development, constrained by low throughput and labor-intensive processes, are being superseded by integrated automated platforms that leverage evolutionary selection. These systems are foundational to biosynthetic pathway elucidation and discovery research, as they enable the systematic exploration of complex sequence-function relationships that are often intractable through rational design alone [57]. By combining industrial-grade automation with continuous directed evolution, researchers can now navigate protein adaptive landscapes and optimize biosynthetic pathways with minimal human intervention, transforming the pace at which high-performance strains for natural product synthesis can be developed [57] [20].
The core of this paradigm shift lies in the implementation of the Design-Build-Test-Learn (DBTL) cycle. Automated biofoundries are facilities dedicated to operationalizing this cycle, integrating computer-aided design, synthetic biology tools, and robotic automation to achieve unprecedented versatility, reproducibility, and scalability in strain engineering [58]. Within this framework, evolutionary selection acts as a powerful discovery engine, identifying optimal enzyme variants and pathway configurations that would be difficult to predict a priori. This technical guide details the components, methodologies, and applications of these integrated platforms, providing a roadmap for researchers engaged in the advanced elucidation and optimization of biosynthetic pathways.
Core Components of Automated Platforms
Automated platforms for strain development are sophisticated robotic systems that integrate various hardware and software components to execute complex biological workflows. A central element is the automated liquid handling robot, such as the Hamilton Microlab VANTAGE or the Tecan Fluent Automation Workstation. These systems are equipped with a central robotic arm to manage labware and integrate off-deck hardware, enabling fully automated protocols for tasks such as transformation set-up, heat shock, washing, and plating [58] [59]. This integration is critical for hands-free operation and significantly enhances throughput, with some systems capable of performing ~400 transformations per day—a ten-fold increase over manual methods [58].
Beyond liquid handling, a fully equipped platform incorporates several specialized modules. Automated colony pickers, like the QPix 460 or the integrated Pickolo, are used to select and transfer transformed clones, ensuring compatibility between the transformation output and downstream cultivation steps [58] [59]. Integrated instruments such as plate sealers, peelers, and thermal cyclers automate the most time-intensive steps of protocols like yeast transformation. Furthermore, positive pressure solid phase extraction systems (e.g., Resolvex M10) and on-deck centrifuges and shakers facilitate automated sample preparation, including plasmid isolation and cell lysis [59]. This modular integration creates a continuous, closed-loop system where the output of one step becomes the direct input for the next, dramatically reducing manual intervention and accelerating the entire DBTL cycle.
Table 1: Key Hardware Components of an Automated Strain Engineering Platform
| Component | Example Model | Primary Function in Workflow |
|---|---|---|
| Automated Workstation | Hamilton Microlab VANTAGE, Tecan Fluent 1080 | Central liquid handling and robotic arm for protocol execution and hardware integration [58] [59]. |
| Colony Picker | QPix 460, Pickolo | Automated selection and transfer of transformed clones for high-throughput culturing [58] [59]. |
| Off-deck Thermocycler | Inheco ODTC | Precise temperature control for heat shock and other incubation steps [58]. |
| Solid Phase Extraction System | Resolvex M10 | Automated preparation and purification of samples, such as plasmid DNA [59]. |
| Microbioreactor System | Not Specified | Enables well-controlled, high-throughput cultivation in microplates with continuous, non-stop shaking [59]. |
Evolutionary Selection Methodologies
Evolutionary selection provides the driving force for optimizing protein function and metabolic pathway flux without requiring comprehensive prior knowledge of sequence-structure relationships. A prominent method for achieving this is continuous directed evolution, such as the OrthoRep system. This system employs orthogonal DNA polymerases to generate random mutations in a target gene of interest at rates above genomic error thresholds, while a genetic circuit links desired protein functions to host cell survival or growth [57]. This growth-coupled selection enables the autonomous exploration of vast sequence spaces, allowing for the evolution of complex functionalities like improved enzyme sensitivity or altered operator selectivity [57].
For screening larger, pre-defined libraries of enzyme variants or homologs, automated high-throughput screening (HTS) is indispensable. The workflow begins with the generation of genetic diversity. This can be achieved through gene diversification techniques like error-prone PCR (epPCR), which uses low-fidelity polymerases to create random mutations, or through the assembly of libraries of homologous genes from different organisms [60]. The automated platform then executes the transformation and cultivation of these libraries into a suitable microbial host, such as Saccharomyces cerevisiae, as previously described [58]. Following growth, a high-throughput chemical extraction method, often based on enzymatic cell lysis (e.g., Zymolyase) followed by organic solvent extraction, is used to prepare metabolite samples [58]. Finally, the analysis is performed using rapid liquid chromatography-mass spectrometry (LC-MS) methods, which are optimized for speed—sometimes reducing runtimes from 50 minutes to under 20 minutes—to enable the efficient quantification of target compound titers across thousands of samples [58] [23]. The entire process, from library transformation to identification of high-performing clones, is orchestrated by the automated platform, ensuring speed, reproducibility, and quantitative rigor.
Quantitative Performance of Automated Evolutionary Platforms
The integration of automation with evolutionary selection yields substantial quantitative gains in the speed, scale, and success of strain engineering campaigns. As highlighted in Table 2, automated platforms can achieve a transformation capacity of approximately 2,000 yeast transformations per week, a ten-fold increase over a manual throughput of roughly 200 per week [58]. This leap in throughput directly translates to a vastly expanded capacity for screening genetic diversity. In practice, screening a library of 32 genes in a verazine-producing yeast strain using an automated pipeline led to the identification of several gene candidates that enhanced the production of this key intermediate by 2.0- to 5-fold [58]. This demonstrates the power of automated HTS to rapidly pinpoint pathway bottlenecks and performance-enhancing genetic elements.
The operational advantages extend beyond raw throughput. Automated systems like the iAutoEvoLab are designed for enhanced reliability and can operate autonomously for extended periods, reported to run for approximately one month with minimal human intervention [57]. This continuous operation is crucial for evolutionary methods that require long-term cultivation and selection pressure. The outcome of these campaigns is the generation of highly optimized biocatalysts. For instance, automated continuous evolution has been successfully used to evolve proteins "from inactive precursors to fully functional entities," such as a T7 RNA polymerase fusion protein with novel mRNA capping properties that can be directly applied in biomedical research [57]. These performance metrics underscore the transformative impact of automation on the scale and efficacy of evolutionary strain development.
Table 2: Performance Metrics of Automated vs. Manual Strain Engineering Workflows
| Performance Metric | Automated Platform | Manual Workflow |
|---|---|---|
| Throughput (Transformations/Week) | ~2,000 [58] | ~200 [58] |
| Operational Duration | Up to ~1 month autonomously [57] | Limited to daily manual operation |
| Typical Fold-Increase Identified | 2.0 to 5.0 [58] | Varies, generally lower due to smaller screen scope |
| Key Outcome | Generation of fully functional proteins from inactive precursors [57] | Labor-intensive, limited exploration of sequence space |
Experimental Protocols for Key Processes
Automated High-Throughput Yeast Transformation
The following protocol is adapted for execution on a Hamilton Microlab VANTAGE system and achieves a throughput of 96 transformations per run [58].
- Workflow Setup: The automated method is divided into three modular steps in the Hamilton VENUS software: "Transformation set up and heat shock," "Washing," and "Plating." Users load the deck with labware according to a predefined layout.
- Transformation Setup: The robot dispenses competent S. cerevisiae cells and plasmid DNA into a 96-well plate. It then adds a lithium acetate/ssDNA/PEG mixture. The precise pipetting parameters for viscous reagents like PEG are pre-optimized for accuracy, adjusting aspiration and dispensing speeds, air gaps, and pre-/post-dispensing parameters [58].
- Heat Shock: The robotic arm transfers the 96-well plate to an off-deck thermal cycler (e.g., Inheco ODTC) for a programmed heat shock incubation. This step is fully automated and hands-free.
- Washing: The plate is returned to the deck, where the system performs centrifugation and washing steps to remove the transformation mixture and resuspend the cells in a suitable buffer.
- Plating: The robot dispenses the transformed cell suspension onto agar plates in a 96-format. The resulting plates, the output of the "Build" step, are compatible with downstream automated colony picking.
High-Throughput Metabolite Extraction and Analysis for Screening
This protocol is designed for the rapid processing of hundreds of yeast cultures to quantify pathway product titers [58].
- Cell Lysis: Transfer a small aliquot of cultured cells (from a 96-deep-well plate) to a new assay plate. Add Zymolyase to enzymatically degrade the yeast cell wall and facilitate lysis.
- Metabolite Extraction: Add an organic solvent (e.g., ethyl acetate or methanol) to the lysate to extract hydrophobic metabolites. The automated system can mix and separate phases if needed.
- Sample Analysis: Inject the processed sample into an LC-MS system. Utilize a rapid LC method that reduces the analytical runtime—for example, from 50 minutes to 19 minutes—to enable high-throughput quantification [58].
- Data Processing: Quantify the titer of the target metabolite (e.g., verazine) by integrating the peak area and comparing it to a standard curve. Normalize titers to optical density or cell count to account for cultural variations.
The Scientist's Toolkit: Essential Research Reagents and Materials
The successful implementation of automated strain engineering relies on a suite of specialized reagents and molecular tools. The table below details key solutions used in the featured experiments and broader field.
Table 3: Key Research Reagent Solutions for Automated Strain Development
| Reagent/Material | Function in Workflow | Example Use Case |
|---|---|---|
| pESC-URA Plasmid | An episomal expression vector for S. cerevisiae with a URA3 auxotrophic marker and inducible GAL1 promoter [58]. | Used for inducible overexpression of library genes in a verazine-producing yeast strain [58]. |
| Zymolyase | An enzyme mixture with β-1,3-glucanase activity that digests the cell wall of yeast and other fungi [58]. | Essential for efficient cell lysis in high-throughput chemical extraction protocols prior to metabolite analysis [58]. |
| NucleoSpin 96 Plasmid Kit | A commercial kit for the high-throughput purification of plasmid DNA from bacterial cultures [59]. | Used in automated workflows on platforms like the Tecan Fluent with the Resolvex M10 system for hands-free plasmid preparation [59]. |
| OrthoRep System | A continuous evolution system featuring an orthogonal DNA polymerase that mutates a target plasmid independently of the host genome [57]. | Enables long-term, continuous directed evolution of proteins in vivo with growth-coupled selection [57]. |
| Hamilton VENUS Software | The proprietary software for programming and controlling Hamilton robotic liquid handling systems [58]. | Allows customization of experimental parameters (e.g., DNA volume, incubation times) and full automation of the transformation protocol [58]. |
The confluence of automated platforms and evolutionary selection represents a cornerstone technology for biosynthetic pathway elucidation and discovery research. By integrating industrial-grade automation with growth-coupled selection and high-throughput screening, these systems enable a systematic and scalable approach to engineering high-performance microbial strains. The detailed methodologies and performance data outlined in this guide provide a framework for researchers to implement and leverage these powerful technologies. As these platforms continue to evolve with advancements in machine learning and deeper integration with multi-omics data, they will further accelerate the development of robust microbial cell factories, paving the way for the sustainable and efficient production of valuable natural products and therapeutics.
Validating and Comparing Pathways: From Enzyme Function to Industrial Viability
In Vitro and In Vivo Enzyme Assays for Functional Characterization
Functional characterization of enzymes through well-designed assays is a cornerstone of modern biosynthetic pathway elucidation and drug discovery research. These assays provide critical insights into enzyme activity, kinetics, specificity, and processivity, enabling researchers to validate putative pathway genes, understand metabolic networks, and identify potential therapeutic targets. In the context of biosynthetic pathway discovery—particularly for valuable plant natural products like hydroxysafflor yellow A (HSYA)—the integration of both in vitro and in vivo approaches has proven essential for comprehensively elucidating complex metabolic routes [23] [17]. The strategic combination of these methodologies allows researchers to bridge the gap between simplified biochemical systems and physiologically relevant cellular environments, ultimately providing a more complete understanding of enzyme function within biological systems.
This technical guide examines established and emerging platforms for enzyme functional characterization, with emphasis on assay design principles, methodological considerations, and practical applications in biosynthetic pathway discovery. We present detailed protocols, analytical frameworks, and experimental workflows that support rigorous enzyme characterization, enabling researchers to select appropriate assay formats based on their specific research objectives, available equipment, and biological context.
Core Concepts and Definitions
Enzyme Activity and Units
Standardized measurement and reporting of enzyme activity are fundamental for meaningful data interpretation and cross-comparison between studies. Unfortunately, inconsistent terminology and unit definitions can significantly complicate these efforts [61].
Table 1: Key Definitions in Enzyme Assays
| Term | Definition | Importance |
|---|---|---|
| Enzyme Unit (U) | Amount of enzyme catalyzing conversion of 1 μmol (Definition A) or 1 nmol (Definition B) of substrate per minute under standard conditions [61] | Critical to specify which definition is used, as values differ 1000-fold |
| Enzyme Activity | Concentration of enzyme units, expressed as U/mL (nmol/min/mL if using Definition B) [61] | Determines volume of enzyme solution needed for assays |
| Specific Activity | Enzyme units per mg of total protein (U/mg or nmol/min/mg) [61] | Key indicator of enzyme purity and quality; should be consistent between batches of pure enzyme |
| Enzymatic Purity | Fraction of observed activity in an assay derived from a single enzyme [62] | Essential for screening; high mass purity doesn't guarantee enzymatic purity |
Assay Validation and Quality Control
Ensuring enzyme preparation quality is paramount for generating reliable data. Enzyme identity confirmation through mass spectrometry and mass purity assessment via SDS-PAGE are essential first steps [62]. However, these alone do not guarantee enzymatic purity—the fraction of observed activity deriving solely from your target enzyme [62].
Signs of enzymatic contamination include abnormal kinetic parameters (Km values not matching literature), biphasic or shallow inhibitor IC50 curves, inability to reach complete inhibition, and irreproducible activities between batches or assay formats [62]. Each new enzyme batch requires validation, as purification variability can introduce contaminating activities that compromise screening campaigns and lead to misleading hit identification [62].
In Vitro Enzyme Assays
In vitro assays utilize purified enzyme preparations and defined reaction conditions to study enzymatic activity directly, enabling precise control of experimental variables and detailed mechanistic studies.
Core Principles and Optimization
Successful in vitro assay implementation requires careful attention to linear range determination, substrate concentration optimization, and appropriate controls.
Operating in the Linear Range: Assay signals must be proportional to enzyme concentration for accurate quantification. This typically requires maintaining substrate conversion below 15% while ensuring sufficient product for detection [61]. As illustrated in Figure 1, signal response becomes non-linear at high enzyme concentrations due to substrate depletion, product inhibition, or detector limitations.
Substrate Concentration: The initial substrate concentration should generally be at least 10-fold higher than the product concentration needed for adequate detection signals. Consideration of the enzyme's Km for the substrate is also important for designing kinetically appropriate assays [61].
Temperature and Time Optimization: Most assays run between 20-37°C for 15-60 minutes. Higher temperatures increase activity but may compromise stability. Very short incubation times (<2 minutes) are discouraged due to timing inaccuracies having disproportionate effects [61].
Specialized In Vitro Assay Formats
Different enzyme classes and research questions require tailored assay methodologies with specific detection strategies.
Processivity and DNA Scanning Assays: For DNA-modifying enzymes like AID/APOBEC deaminases, specialized assays measure both catalytic activity and processive scanning behavior. Under single-hit conditions using fluorescently labeled ssDNA substrates, these assays quantify facilitated diffusion mechanisms—including one-dimensional sliding and three-dimensional jumping/intersegment transfer—that determine mutagenic potential in vivo [63].
Glycosyltransferase Assays: Glycosyltransferases present particular challenges as they typically don't produce directly detectable products. Common solutions include coupled-enzyme assays that detect nucleotide byproducts (NDP or CMP), or HPLC-based methods with fluorescent substrates for sensitive product quantification [64]. The diversity of GT substrates and mechanisms has necessitated developing numerous specialized approaches, with selection depending on the specific project requirements and enzyme characteristics [64].
Table 2: In Vitro Assay Methods for Glycosyltransferases
| Method | Principle | Applications | Considerations |
|---|---|---|---|
| Coupled-Enzyme | Detection of NDP/CMP byproducts via secondary enzymes [64] | General screening, kinetics | Potential interference from coupling enzymes |
| HPLC with Fluorescence | Separation and quantification of fluorescently labeled products [64] | Specific activity, substrate profiling | Lower throughput, requires specialized equipment |
| Capillary Electrophoresis | Separation of charged products in capillary format [64] | Process monitoring, mechanistic studies | Method development complexity |
| Mass Spectrometry | Direct detection of product mass [64] | Uncharacterized reactions, substrate promiscuity | Quantitative challenges, equipment cost |
In Vivo Enzyme Assays
In vivo enzyme assays provide functional characterization within cellular environments, preserving native context including subcellular localization, cofactor availability, and potential regulatory interactions.
Heterologous Expression Systems
Reconstituting biosynthetic pathways in heterologous hosts provides powerful platforms for gene function validation and natural product production.
Plant-Based Systems: Transient expression in Nicotiana benthamiana enables rapid testing of candidate genes and pathway reconstitution. This approach was instrumental in elucidating the HSYA biosynthetic pathway, where co-expression of CtF6H (flavanone 6-hydroxylase), CtCGT (C-glycosyltransferase), Ct2OGD1 (dioxygenase), and CtCHI1 (isomerase) demonstrated complete pathway functionality [23].
Microbial Platforms: Engineered yeast strains offer robust systems for pathway assembly and optimization. Semi-synthesis in yeast enabled characterization of intermediate steps in HSYA biosynthesis and provided a production platform for this valuable compound [23].
Live Bacterial Enzyme Activity Profiling
The Live E. coli Assay (LEICA) platform represents a innovative approach for studying human metabolic enzymes and their genetic variants in a cellular context. By replacing specific E. coli metabolic genes with human orthologs, bacterial growth directly correlates with human enzyme activity [65].
This platform has successfully characterized mutations in human glucose-6-phosphate isomerase (GPI) associated with hemolytic anemia and glucose-6-phosphate dehydrogenase (G6PD) variants causing enzymopathies [65]. Growth rates of humanized E. coli strains showed high linear correlation with biochemically determined enzyme activities (R² = 0.84 for G6PD), enabling rapid functional screening of sequence variants [65]. LEICA also facilitates drug discovery, as demonstrated by identification of G6PD inhibitors and agonists through bacterial growth modulation [65].
Gene Silencing and Functional Validation
Virus-Induced Gene Silencing (VIGS) in native hosts provides critical in vivo validation of gene function. In safflower, silencing CtCGT and CtF6H reduced HSYA accumulation by approximately 30%, directly implicating these genes in the biosynthetic pathway [23]. This approach preserves native cellular environments and regulatory contexts, complementing heterologous expression studies.
Integrated Approaches for Pathway Elucidation
Elucidating complete biosynthetic pathways requires strategic integration of multiple methodologies to build comprehensive understanding of metabolic networks.
Biosynthetic Pathway Case Study: Hydroxysafflor Yellow A
The recent characterization of the HSYA pathway exemplifies this integrated approach. Researchers combined:
- Co-expression analysis of transcriptome data from different tissues and developmental stages [23]
- Phylogenetic analysis to identify candidate C-glycosyltransferases and P450 enzymes [23]
- Enzyme characterization through heterologous expression and in vitro assays [23]
- Pathway validation via VIGS and heterologous reconstruction [23]
This multi-platform strategy revealed four key enzymes: CtF6H (P450 hydroxylase), CtCHI1 (isomerase), CtCGT (di-C-glycosyltransferase), and Ct2OGD1 (dioxygenase) that coordinately convert naringenin to HSYA [23]. The specific combination and high expression of these genes, along with absence of competing F2H activity, explains HSYA's unique occurrence in safflower [23].
Advanced Systems Biology Approaches
Emerging methodologies are enhancing our ability to decipher complex plant metabolic pathways:
- Co-expression analysis identifies coordinately regulated genes suggesting functional relationships [17]
- Gene cluster identification reveals physically linked biosynthetic genes [17]
- Metabolite profiling correlates metabolite abundance with gene expression [17]
- Deep learning approaches predict enzyme function and pathway associations [17]
- Genome-wide association studies link genetic variation to metabolic traits [17]
- Protein complex identification reveals metabolons that channel pathway intermediates [17]
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents for Enzyme Functional Characterization
| Reagent/Material | Function | Application Examples |
|---|---|---|
| Color-Coded Specimen Vials [66] [67] | Sample tracking and organization; standardized color codes indicate content type | Lavender/purple for EDTA blood samples; light blue for coagulation studies [67] |
| Pre-labeled Cryovials [66] | Maintain sample identity under cryogenic conditions; prevent Sharpie fading at -80°C | Long-term storage of enzyme preparations or tissue samples [66] |
| UDP-sugars [23] [64] | Glycosyl donor substrates for glycosyltransferase assays | UDP-glucose for CtCGT in HSYA biosynthesis [23] |
| NADPH [23] | Cofactor for cytochrome P450 enzymes | CtF6H hydroxylation reactions in HSYA pathway [23] |
| Enzyme Inhibitor Cocktails [62] | Suppress contaminating activities in enzyme preparations | Protease/phosphatase inhibitors for maintaining enzymatic purity [62] |
| Fluorescently Labeled DNA [63] | Substrates for processivity and DNA scanning assays | AID/APOBEC deamination assays on ssDNA [63] |
Comprehensive functional characterization of enzymes demands strategic implementation and integration of both in vitro and in vivo assay platforms. In vitro systems provide precise mechanistic insights under controlled conditions, while in vivo approaches capture physiological context and complexity. The continuing development of innovative technologies—including coupled enzyme assays, humanized microbial platforms, and advanced systems biology approaches—is expanding our capability to decipher complex metabolic pathways and accelerate natural product discovery. As these methodologies evolve, they will undoubtedly yield new insights into enzyme function and enable more efficient engineering of biosynthetic pathways for therapeutic applications.
Metabolite profiling represents a cornerstone of modern biosynthetic pathway elucidation and discovery research, providing critical insights into the complex networks of small molecules that underpin biological systems. As the functional readout of cellular processes, metabolites offer a direct window into biochemical activity, making their comprehensive analysis indispensable for understanding natural product biosynthesis, identifying novel therapeutic compounds, and advancing drug development. The integration of advanced analytical technologies has transformed metabolite profiling from simple compound identification to sophisticated systems-level analysis, enabling researchers to decode biosynthetic pathways with unprecedented precision [45] [68].
This technical guide examines the three principal analytical platforms—Liquid Chromatography-Mass Spectrometry (LC-MS), Nuclear Magnetic Resonance (NMR) spectroscopy, and Gas Chromatography-Mass Spectrometry (GC-MS)—that form the foundation of contemporary metabolomics research. Within the context of biosynthetic pathway elucidation, each technique offers unique capabilities for characterizing metabolite structures, quantifying pathway intermediates, and reconstructing biochemical networks. The complementary nature of these platforms provides researchers with a powerful toolkit for addressing the complex challenges of metabolite identification, pathway mapping, and natural product discovery [69] [70].
Core Analytical Platforms: Technical Principles and Applications
Liquid Chromatography-Mass Spectrometry (LC-MS)
Technical Principles: LC-MS combines the superior separation capabilities of liquid chromatography with the high sensitivity and detection power of mass spectrometry. The technique typically employs reverse-phase chromatography using C18 columns with mobile phases consisting of water and acetonitrile, both modified with 0.1% formic acid to enhance ionization [71] [69]. Modern systems utilize ultra-high-performance liquid chromatography (UHPLC) that operates at significantly higher pressures, improving resolution and reducing analysis times to 2-5 minutes per sample [69].
Mass detection commonly employs high-resolution accurate mass (HRAM) instruments such as Q-Exactive Orbitrap, quadrupole-time-of-flight (Q-TOF), and triple quadrupole (QQQ) mass analyzers [71] [69]. Ionization is primarily achieved through electrospray ionization (ESI) operating in both positive and negative modes, though atmospheric pressure chemical ionization (APCI) and atmospheric pressure photoionization (APPI) extend the range of analyzable compounds [69] [68].
Applications in Pathway Elucidation: LC-MS has become the dominant technology for untargeted metabolomics in biosynthetic pathway research due to its ability to detect a broad spectrum of nonvolatile hydrophobic and hydrophilic metabolites without derivatization [69]. It enables researchers to perform comprehensive metabolite discovery from crude natural extracts while simultaneously conducting pathway-specific investigations [45]. A recent study demonstrated its power in elucidating the complete biosynthetic pathway of hydroxysafflor yellow A (HSYA), where LC-MS analysis confirmed the unique presence of this valuable quinochalcone in safflower flowers and facilitated the identification of key intermediates [23].
Table 1: LC-MS Instrumentation Parameters for Metabolite Profiling
| Parameter | Typical Configuration | Pathway Elucidation Application |
|---|---|---|
| Chromatography | UHPLC with C18 column (100 × 2.1 mm, 1.8 μm) | Separation of complex natural extracts |
| Mobile Phase | Water/Acetonitrile + 0.1% Formic Acid | Resolution of polar and non-polar intermediates |
| Mass Analyzer | Q-TOF, Orbitrap, Triple Quadrupole | High-mass accuracy for unknown identification |
| Mass Range | 100-1200 m/z | Coverage of primary and secondary metabolites |
| Resolution | 70,000 (full scan); 17,500 (MS/MS) | Differentiation of isobaric compounds |
| Ionization | ESI (±), APCI, APPI | Broad metabolite coverage |
Nuclear Magnetic Resonance (NMR) Spectroscopy
Technical Principles: NMR spectroscopy exploits the magnetic properties of certain atomic nuclei (most commonly ¹H, ¹³C, ¹⁵N, and ³¹P) when placed in a strong magnetic field. The technique provides detailed structural information through chemical shifts, coupling constants, and integration data [70]. Unlike MS-based methods, NMR requires no separation prior to analysis and is inherently quantitative, as all metabolites are detected with the same sensitivity using a single internal standard [70].
Modern NMR metabolomics employs standardized one-dimensional pulse sequences including ¹H 1D NOESY with water presaturation for aqueous samples and ¹H 1D CPMG for protein-rich biofluids [70]. Recent advancements in hyperpolarization techniques, such as dynamic nuclear polarization (DNP) and parahydrogen-induced polarization (PHIP), have dramatically improved sensitivity—historically NMR's primary limitation compared to MS [70].
Applications in Pathway Elucidation: NMR's exceptional reproducibility and quantitative accuracy make it invaluable for tracking flux through biosynthetic pathways and confirming metabolite structures identified by MS. Its non-destructive nature allows for repeated analysis of precious samples and enables the identification of novel compounds through complete structural elucidation [70]. In plant metabolomics, NMR effectively differentiates chemotypes and quantifies major pathway products, as demonstrated in studies of Tetrastigmae Radix where it complemented MS findings [72] [70].
Gas Chromatography-Mass Spectrometry (GC-MS)
Technical Principles: GC-MS couples the separation power of gas chromatography with the detection capabilities of mass spectrometry, making it particularly suitable for volatile and thermally stable metabolites [73] [68]. Sample preparation typically requires chemical derivatization (e.g., trimethylsilylation, oximation) to increase volatility and thermal stability of polar compounds such as organic acids, amino acids, and sugars [68].
Separation occurs in a high-temperature oven using capillary columns with stationary phases of varying polarity. Electron ionization (EI) at 70 eV is the most common ionization method, producing reproducible fragmentation patterns that can be matched against extensive spectral libraries [68]. Advanced configurations including two-dimensional GC (GC×GC) coupled to time-of-flight (TOF) mass analyzers significantly enhance separation capacity and compound identification [68].
Applications in Pathway Elucidation: GC-MS excels in profiling primary metabolites central to core metabolic pathways, including carbohydrates, organic acids, and amino acids [73]. Its application in tracing carbon flux through central carbon metabolism provides critical information for understanding pathway regulation and engineering efforts [73]. The technique's high chromatographic resolution and extensive, searchable spectral libraries make it particularly valuable for identifying known pathway intermediates and diagnosing metabolic bottlenecks in engineered systems [74].
Table 2: Comparative Analysis of Metabolite Profiling Techniques
| Parameter | LC-MS | NMR | GC-MS |
|---|---|---|---|
| Sensitivity | nM-fM range [69] | μM-nM range [70] | pM-nM range [68] |
| Sample Throughput | High | Moderate | Moderate to High |
| Metabolite Coverage | Broad (polar to non-polar) | Broad (detectable nuclei) | Volatile/derivatizable compounds |
| Quantitation | Relative (requires standards) | Absolute (internal standard) [70] | Relative (requires standards) |
| Structural Elucidation | MS/MS fragmentation | Complete structure determination | Library matching (EI spectra) |
| Sample Preparation | Moderate | Minimal | Extensive (derivatization) |
| Reproducibility | Good | Excellent [70] | Good |
| Key Strength | Sensitivity and breadth | Structure elucidation and quantitation | Library searchability and resolution |
Integrated Workflows for Biosynthetic Pathway Elucidation
Elucidating complete biosynthetic pathways requires the strategic integration of multiple analytical platforms to leverage their complementary strengths. A representative workflow begins with untargeted LC-MS analysis to comprehensively profile crude extracts and identify candidate pathway metabolites through high-resolution mass measurement and MS/MS fragmentation [45] [71]. NMR then provides definitive structural confirmation of key intermediates, particularly for novel compounds not present in databases [70]. GC-MS profiles primary metabolic precursors and cofactors, establishing connections to central metabolism [73] [74].
This multi-platform approach was successfully applied in deciphering the biosynthetic pathway of hydroxysafflor yellow A, where LC-MS first identified HSYA's unique presence in safflower flowers, followed by NMR-assisted structural verification of intermediates, and GC-MS analysis of central carbon metabolites that feed into the pathway [23]. The integrated data enabled researchers to characterize four key biosynthetic enzymes—CtF6H (flavanone 6-hydroxylase), CtCHI1 (chalcone-flavanone isomerase), CtCGT (flavonoid di-C-glycosyltransferase), and Ct2OGD1 (2-oxoglutarate-dependent dioxygenase)—that collectively convert naringenin to HSYA [23].
Figure 1: Integrated Workflow for Pathway Elucidation. This diagram outlines the multi-technique approach to decoding biosynthetic pathways, from initial discovery through final validation.
Experimental Protocols for Pathway Elucidation
Untargeted Metabolomics for Novel Pathway Discovery
Sample Preparation:
- Plant Materials: Fresh tissues (100 mg) are flash-frozen in liquid nitrogen and homogenized with steel balls at 60 Hz for 2 minutes [71].
- Extraction: Add 1 mL of cold methanol/water (7:3, v/v) containing internal standard (2-chloro-L-phenylalanine, 0.06 mg/mL), vortex, sonicate in ice water bath for 30 minutes, and incubate at -20°C overnight [71].
- Processing: Centrifuge at 13,000 × g for 10 minutes at 4°C, collect 150 μL supernatant, filter through 0.22 μm membrane, and transfer to LC vials [71].
LC-MS Analysis:
- Chromatography: ACQUITY UPLC HSS T3 column (100 × 2.1 mm, 1.8 μm); column temperature: 45°C; flow rate: 0.35 mL/min; mobile phase A: water + 0.1% formic acid, B: acetonitrile + 0.1% formic acid [71].
- Gradient: 5% B (0-2 min), 5-30% B (2-4 min), 30-50% B (4-8 min), 50-80% B (8-10 min), 80-100% B (10-14 min), 100% B (14-15 min), 100-5% B (15-15.1 min), 5% B (15.1-16 min) [71].
- Mass Spectrometry: ESI positive/negative mode; capillary temperature: 320°C; spray voltage: ±3.8 kV; sheath gas: 35 Arb; mass range: 100-1200 m/z; resolution: 70,000 (full scan), 17,500 (MS/MS) [71].
Data Processing:
- Use software such as Progenesis QI for peak picking, alignment, and deconvolution with parameters: precursor tolerance 5 ppm, product ion threshold 5% [71].
- Multivariate statistical analysis (PCA, OPLS-DA) in SIMCA or similar platforms; metabolites with VIP >1, p<0.05, and fold change ≥2 are considered significant [71] [72].
- Pathway enrichment analysis using KEGG or MetaboAnalyst databases [71].
NMR-Based Structural Elucidation
Sample Preparation:
- Metabolite Extraction: Prepare samples using methanol-chloroform-water extraction for comprehensive metabolite coverage [70].
- Buffer Conditions: Use phosphate buffer (100 mM, pH 7.4) in D₂O containing 0.5-1.0 mM TSP (sodium trimethylsilylpropionate) as chemical shift reference [70].
- Volume: Transfer 600 μL of sample to 5 mm NMR tubes [70].
NMR Acquisition:
- Instrumentation: 600 MHz spectrometer with cryoprobe for enhanced sensitivity [70].
- Water Suppression: Employ 1D NOESY presaturation sequence for aqueous samples [70].
- Parameters: Acquisition time: 2-3 seconds; relaxation delay: 1-2 seconds; temperature: 298K; number of scans: 64-128 [70].
- 2D Experiments: Conduct ¹H-¹H COSY, ¹H-¹³C HSQC, and HMBC for complete structural assignment of unknown metabolites [70].
GC-MS for Primary Metabolite Profiling
Sample Derivatization:
- Methoximation: Add 20 μL of methoxyamine hydrochloride (20 mg/mL in pyridine), incubate at 30°C for 90 minutes [73] [68].
- Silylation: Add 32 μL of N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) with 1% trimethylchlorosilane (TMCS), incubate at 37°C for 30 minutes [68].
GC-MS Analysis:
- Chromatography: DB-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness); carrier gas: helium at 1.0 mL/min [68].
- Temperature Program: 60°C (1 min), 60-325°C at 10°C/min, 325°C (10 min) [68].
- Mass Spectrometry: Electron ionization at 70 eV; ion source temperature: 230°C; mass range: 50-600 m/z [68].
Data Processing:
- Peak detection and alignment using AMDIS or similar software [73].
- Compound identification against commercial libraries (NIST, Fiehn) with match factor >800 [68].
- Normalize to internal standards (e.g., ribitol for retention time alignment) [73].
Research Reagent Solutions for Metabolite Profiling
Table 3: Essential Research Reagents for Metabolite Profiling Experiments
| Reagent/Material | Function | Application Notes |
|---|---|---|
| Methanol with Internal Standard | Metabolite extraction and quantification | 2-chloro-L-phenylalanine (0.06 mg/mL) for LC-MS; ribitol for GC-MS [71] [73] |
| Acetonitrile (LC-MS Grade) | Mobile phase for chromatography | With 0.1% formic acid for improved ionization [71] [69] |
| MSTFA with 1% TMCS | Derivatization for GC-MS | Sillyating agent for polar functional groups [68] |
| Deuterated Solvents | NMR lock signal and shimming | D₂O for aqueous samples; CD₃OD for lipid extracts [70] |
| TSP | Chemical shift reference for NMR | Sodium trimethylsilylpropionate; use at 0.5-1.0 mM [70] |
| UDP-Glucose | Cofactor for glycosyltransferase assays | Essential for characterizing enzymes like CtCGT [23] |
| NADPH | Cofactor for cytochrome P450 reactions | Required for hydroxylase activity (e.g., CtF6H) [23] |
Case Study: Elucidating the Hydroxysafflor Yellow A Biosynthetic Pathway
The power of integrated metabolite profiling is exemplified by the recent complete elucidation of the hydroxysafflor yellow A (HSYA) biosynthetic pathway [23]. HSYA is a valuable quinochalcone C-glycoside with demonstrated efficacy in treating acute ischemic stroke that has recently completed phase III clinical trials. Researchers employed a comprehensive multi-omics strategy to decode this complex pathway:
Discovery Phase: Untargeted LC-MS analysis of different safflower tissues revealed HSYA's exclusive accumulation in flowers, providing initial tissue specificity clues [23]. Comparative transcriptomics of budding versus blooming flowers identified co-expressed genes that correlated with HSYA accumulation patterns.
Enzyme Characterization: Functional analysis identified four key biosynthetic enzymes: CtF6H (flavanone 6-hydroxylase) that catalyzes the 6-hydroxylation of naringenin to produce carthamidin; CtCHI1 that isomerizes between carthamidin and isocarthamidin; CtCGT that adds dual C-glucosyl groups; and Ct2OGD1, a 2-oxoglutarate-dependent dioxygenase that completes the quinochalcone formation [23].
Validation: Virus-induced gene silencing (VIGS) of CtCGT and CtF6H in safflower plants resulted in 29.6% and 30.8% reductions in HSYA content, respectively, confirming their in vivo roles [23]. Successful de novo biosynthesis of HSYA in Nicotiana benthamiana provided ultimate validation of the complete pathway.
This case study demonstrates how strategic integration of metabolite profiling technologies with functional genomics enables the decoding of even highly complex plant biosynthetic pathways, opening possibilities for metabolic engineering and heterologous production of valuable natural products.
The synergistic application of LC-MS, NMR, and GC-MS platforms provides an unparalleled toolkit for comprehensive metabolite profiling and biosynthetic pathway elucidation. LC-MS delivers the sensitivity and throughput needed for untargeted discovery, NMR provides the structural rigor required for definitive compound identification, and GC-MS offers the robust quantitative analysis of central metabolic intermediates. As these technologies continue to advance—with improvements in UHPLC resolution, NMR sensitivity through hyperpolarization, and GC×GC comprehensive—their collective power to decode complex biosynthetic networks will only increase.
For researchers engaged in natural product discovery and pathway engineering, the strategic integration of these complementary platforms is no longer optional but essential for success. The workflow outlined in this guide, from initial untargeted profiling to final pathway validation, provides a roadmap for efficiently navigating the complex landscape of metabolic network elucidation. As metabolomics continues to evolve toward more integrated multi-omics approaches, these foundational analytical techniques will remain central to unlocking nature's chemical diversity for drug development and biotechnology applications.
Comparative Analysis of Pathway Efficiency Across Different Host Organisms
The elucidation and engineering of biosynthetic pathways are fundamental to advancing the sustainable production of high-value chemicals, from pharmaceuticals to food additives [21] [75]. However, transferring a pathway from its native organism to a heterologous host like E. coli or yeast does not guarantee efficient function. The metabolic burden, suboptimal enzyme kinetics, and incompatibility with the host's native metabolism can drastically reduce yield [76]. Therefore, a systematic comparison of pathway efficiency across different host organisms is a critical step in bioproduction pipeline. This analysis, framed within the broader context of biosynthetic pathway discovery, enables researchers to identify the most suitable chassis organism and pinpoint necessary engineering strategies to maximize titer, rate, and yield (TRY) [75]. This whitepaper provides an in-depth technical guide for conducting such a comparative analysis, detailing key metrics, computational and experimental methodologies, and data interpretation for an audience of researchers, scientists, and drug development professionals.
Key Metrics for Evaluating Pathway Efficiency
Evaluating pathway efficiency requires a multi-faceted approach that considers stoichiometry, thermodynamics, and cellular physiology. The following metrics are indispensable for a meaningful comparative analysis.
Table 1: Key Quantitative Metrics for Pathway Efficiency Analysis
| Metric Category | Specific Metric | Description & Significance | Ideal Value/Range |
|---|---|---|---|
| Stoichiometric & Yield | Theoretical Yield | Maximum moles of target product per mole of substrate, based on biochemical stoichiometry. Sets the upper limit for performance [75]. | Pathway-dependent; higher is better. |
| Actual Yield | Experimentally measured yield. The ratio of Actual to Theoretical Yield indicates pathway optimization potential. | As close to Theoretical Yield as possible. | |
| Carbon Efficiency | Percentage of carbon from the substrate that is incorporated into the target product. Critical for economic viability [75]. | >80% for highly efficient pathways. | |
| Kinetic & Productivity | Volumetric Productivity | Amount of product formed per unit volume of culture per unit time (e.g., g/L/h). Crucial for bioreactor scaling [76]. | Industry and product-dependent; higher is better. |
| Specific Productivity | Amount of product formed per unit cell mass per unit time (e.g., g/gDCW/h). Normalizes for cell growth. | Industry and product-dependent; higher is better. | |
| Maximum Specific Growth Rate (μₘₐₓ) | Host's growth rate without pathway expression. A significant reduction indicates high metabolic burden. | Minimize difference from host μₘₐₓ. | |
| Thermodynamic & Enzymatic | Pathway Thermodynamic Feasibility | Overall Gibbs free energy change (ΔG) of the pathway. A significantly positive value indicates infeasibility [75]. | Negative or near-zero. |
| Enzyme Abundance & Turnover | Measured via proteomics and enzyme kinetics (kcat/KM). Identifies possible "bottleneck" enzymes [21]. | High abundance and turnover for all steps. | |
| Host-Pathway Integration | Cofactor/Cosubstrate Balance | Regeneration of ATP, NADPH, etc. Imbalance can halt production and stress the host [75] [76]. | Balanced consumption and regeneration. |
| Byproduct Spectrum & Toxicity | Identification and quantification of secreted byproducts (e.g., acetate). Can inhibit growth and production [76]. | Minimal toxic byproduct formation. |
Methodologies for Comparative Analysis
A robust comparison integrates computational predictions with rigorous experimental validation. The following protocols outline a standardized workflow.
Computational Modeling andIn SilicoPrediction
Computational tools allow for the rapid screening of hosts and pathway designs before moving to the lab.
Protocol 1: Genome-Scale Metabolic Modeling (GEM) with Constraint-Based Optimization
- Model Selection: Obtain high-quality, organism-specific GEMs (e.g., iML1515 for E. coli, iMM904 for S. cerevisiae).
- Pathway Integration: Incorporate the heterologous pathway reactions into the GEMs of all candidate hosts (e.g., E. coli, S. cerevisiae, B. subtilis). Ensure all reactions are elementally and charge-balanced [75].
- Simulation Setup: Use Flux Balance Analysis (FBA) to simulate growth and production under defined conditions (e.g., minimal glucose media). Set the objective function to maximize biomass or product synthesis.
- In Silico Analysis:
- Perform Gene Knockout Simulations to identify potential host-specific knockouts that enhance yield [76].
- Use Parsimonious FBA (pFBA) to predict flux distributions that maximize yield while minimizing enzyme investment.
- Calculate the Maximum Theoretical Yield for each host-pathway combination.
- Tool Recommendation: The SubNetX algorithm is particularly effective for this, as it extracts and ranks balanced biosynthetic pathways from biochemical databases and integrates them into GEMs, ensuring stoichiometric feasibility from multiple precursors [75].
Protocol 2: Dynamic Kinetic Modeling of Host-Pathway Interactions
- Model Construction: Develop a kinetic model for the heterologous pathway, incorporating enzyme kinetics and regulatory mechanisms.
- Host Integration: Couple this kinetic model with the host's GEM. A novel strategy uses surrogate machine learning models to replace FBA calculations, dramatically speeding up dynamic simulations [76].
- Simulation: Predict time-course profiles of metabolite accumulation, nutrient depletion, and byproduct formation under different genetic perturbations or carbon sources [76].
- Application: This method is ideal for screening dynamic control circuits and optimizing feeding strategies in bioreactors.
Experimental Validation and Characterization
Computational predictions must be validated experimentally. The following protocols ensure consistent, comparable data across hosts.
Protocol 3: Standardized Fermentation and Metabolite Analysis
- Strain Engineering: Construct pathway expression cassettes using a standardized vector system (e.g., pET, pRSF for E. coli; pRS for S. cerevisiae) to ensure consistent gene dosage and promoter strength across hosts. Use Golden Gate or Gibson Assembly for modular cloning.
- Cultivation: Cultivate all engineered strains and wild-type controls in parallel, using controlled bioreactors to maintain consistent pH, temperature, and dissolved oxygen. Use defined minimal media to enable accurate carbon balancing.
- Sampling: Take periodic samples throughout the growth phase for analysis.
- Analytics:
- Cell Density: Measure optical density (OD600).
- Metabolites: Use LC-MS/MS or GC-MS to quantify the target product, key pathway intermediates, and major byproducts (e.g., acetate, lactate, ethanol) from the culture supernatant [21] [17].
- Substrates: Measure residual substrate (e.g., glucose) concentration.
- Data Calculation: From the analytics data, calculate the metrics listed in Table 1, including volumetric productivity, specific productivity, and yields.
Protocol 4: Multi-Omics Analysis for Bottleneck Identification
- Sample Preparation: Harvest cells from the mid-exponential growth phase from both the engineered and control strains.
- Transcriptomics: Perform RNA-Seq to analyze global gene expression changes. Identify co-expression networks and differentially expressed genes, particularly in the host's native metabolism that interacts with the heterologous pathway [21].
- Proteomics: Conduct LC-MS/MS-based proteomics to quantify enzyme abundance levels for both heterologous and key host proteins. This directly identifies if a pathway enzyme is poorly expressed.
- Fluxomics: Use ¹³C isotopic labeling (e.g., with [U-¹³C]-glucose) and measure isotopic enrichment in metabolites via MS. This provides an experimental measure of in vivo metabolic flux, which can be compared to computational predictions [75].
The following workflow diagram summarizes the integrated computational and experimental approach for comparative pathway analysis.
The Scientist's Toolkit: Essential Research Reagents and Solutions
Successful pathway analysis relies on a suite of specialized reagents, databases, and software tools.
Table 2: Key Research Reagent Solutions for Pathway Analysis
| Category | Item | Function & Application |
|---|---|---|
| Cloning & Expression | Modular Vector Systems (e.g., MoClo, Golden Gate) | Enables rapid, standardized assembly of multi-gene pathways across different hosts. |
| Agrobacterium-mediated Transient Expression (for plants) | Allows rapid, simultaneous co-expression of multiple genes in Nicotiana benthamiana for functional characterization [21]. | |
| Analytical Standards | Stable Isotope-Labeled Standards (¹³C, ¹⁵N) | Essential for quantitative mass spectrometry in metabolomics and fluxomics for accurate concentration and flux determination. |
| Authentic Chemical Standards | Pure samples of the target product and pathway intermediates are required for developing and calibrating analytical methods (LC-MS/GC-MS). | |
| Database & Software | Biochemical Databases (KEGG, MetaCyc, ARBRE, ATLASx) | Provide curated and predicted reaction networks for in silico pathway discovery and extraction [77] [75]. |
| Pathway Modeling Tools (PathVisio, CellDesigner) | Used to create, visualize, and annotate pathway models in standard formats (SBGN, SBML) for sharing and analysis [77]. | |
| Genome-Scale Modeling Platforms (CobraPy, RAVEN) | Software toolboxes for constraint-based modeling, simulation, and analysis of metabolic networks in platforms like MATLAB or Python. |
Data Interpretation and Strategy Formulation
The final step involves synthesizing data from all previous stages to make informed decisions.
- Identifying the Optimal Host: The host with the highest actual yield and productivity, minimal growth impairment, and the most favorable byproduct profile (as per Table 1) is typically the lead candidate. Computational predictions from SubNetX and GEMs should align with these experimental findings [75].
- Diagnosing Host-Specific Bottlenecks:
- Low Enzyme Expression: Addressed by optimizing codon usage, using stronger promoters, or engineering enzyme stability.
- Cofactor Imbalance: Solved by engineering cofactor regeneration systems or using enzyme variants with alternative cofactor specificity.
- Toxic Intermediate Accumulation: Mitigated by fusing enzymes to form metabolons or fine-tuning the expression of upstream and downstream enzymes [17].
- Suboptimal Host Metabolism: Addressed through gene knockouts or overexpression of native genes to redirect carbon flux, as predicted by GEMs.
- Designing Engineering Strategies: Based on the diagnostic, an iterative engineering cycle (Design-Build-Test-Learn) is initiated. The comparative data provides a clear rationale for which modifications are most likely to succeed in a given host organism.
A systematic comparative analysis of pathway efficiency is not a mere preliminary step but a continuous, iterative process that deeply informs the entire metabolic engineering workflow. By integrating sophisticated computational predictions from tools like SubNetX with rigorous, multi-omics-guided experimental validation, researchers can move beyond simple pathway expression to true pathway optimization. This approach enables the rational selection of the most efficient host organism and the precise identification of host-specific bottlenecks, ultimately paving the way for the development of robust microbial cell factories for the sustainable production of complex and valuable chemicals.
In the field of industrial biotechnology and pharmaceutical development, the successful elucidation of a biosynthetic pathway is merely the first step toward commercialization. The true measure of success lies in translating this discovery into a viable manufacturing process, a task that relies heavily on the precise quantification of key performance indicators. Titer, yield, and productivity serve as the fundamental triad of metrics that bridge the gap between laboratory-scale pathway discovery and industrial-scale production. These parameters provide the critical data needed to assess economic feasibility, optimize bioprocess conditions, and scale up production of valuable compounds such as the investigational stroke drug Hydroxysafflor Yellow A (HSYA) and other plant-derived therapeutics [23].
Within the broader context of biosynthetic pathway elucidation, these metrics validate not only the efficiency of the engineered organism or system but also the functional completeness of the discovered pathway. As research increasingly leverages big data, multi-omics analyses, and advanced computational tools to unravel complex plant metabolic pathways, the resulting insights must ultimately be quantified through these industrial performance measures [21]. This guide provides researchers and drug development professionals with a technical framework for measuring, benchmarking, and optimizing these critical metrics in an industrial biosynthetic context.
Defining the Core Metrics
The trilogy of titer, yield, and productivity provides a comprehensive picture of bioprocess performance, with each metric offering a distinct perspective on efficiency and effectiveness. Understanding their specific definitions, calculations, and interrelationships is fundamental to accurate process evaluation.
Titer: Titer refers to the concentration of the target product accumulated in the fermentation broth or reaction vessel at the conclusion of the process. It is typically expressed in units of grams per liter (g/L) and represents the final output capacity of the production system. While a high titer is desirable, it does not account for the time invested or the resources consumed.
Yield: Yield measures the efficiency of substrate conversion into the desired product. It can be expressed as gravimetric yield (grams of product per gram of substrate) or molar yield (moles of product per mole of substrate). This metric is crucial for evaluating the economic and resource efficiency of the process, as it directly impacts raw material costs and waste generation.
Productivity: Productivity, often termed volumetric productivity, represents the rate of product formation per unit volume per unit time. It is calculated as the titer divided by the total process time and expressed as g/L/h. This metric is particularly important in an industrial context as it reflects the throughput and capital efficiency of production facilities, directly influencing manufacturing capacity and cost.
Table 1: Core Bioprocess Performance Metrics
| Metric | Definition | Typical Units | Significance |
|---|---|---|---|
| Titer | Concentration of product at process end | g/L | Measures output capacity |
| Yield | Efficiency of substrate conversion to product | g product/g substrate | Measures resource utilization |
| Productivity | Rate of product formation | g/L/h | Measures production speed & facility throughput |
These metrics are interrelated; improvements in one often impact the others. For instance, strategies to increase titer may sometimes reduce productivity if they require longer fermentation times, while yield improvements typically enhance both titer and productivity by making more efficient use of substrates. The optimal balance depends on specific economic and operational constraints.
Quantitative Benchmarking in Industrial and Research Contexts
Establishing realistic performance targets requires understanding current industry benchmarks and research achievements. These benchmarks vary significantly across biological systems, product classes, and technological maturity levels, providing crucial context for evaluating the commercial potential of newly elucidated biosynthetic pathways.
Cross-Industry Productivity Benchmarks
Recent comprehensive analyses of workforce productivity offer valuable parallels for industrial bioprocess optimization. The 2025 Productivity Benchmarks Report from ActivTrak, which aggregated data from 774 companies and nearly 219,000 employees, revealed significant variation in productive time and work patterns across sectors [78]. The logistics sector led with 7 hours and 3 minutes of daily productive time, while financial services and insurance followed with approximately 6.5 hours [78]. These benchmarks highlight the importance of sector-specific performance standards, a concept that directly translates to industrial biotechnology where different product categories (therapeutics, biofuels, specialty chemicals) have distinct efficiency expectations.
The same study revealed that industries with the highest technology adoption, such as logistics where 72% of workers use AI tools, demonstrated superior productivity metrics [78]. This correlation mirrors the biomanufacturing sector, where advanced analytical technologies and process controls typically drive higher titers and productivities. Additionally, the report noted that remote-only workers showed the highest daily productivity (+29 minutes versus other location types), suggesting that operational structure and environment significantly impact output efficiency—a consideration relevant to bioprocess design and scale-up strategies [78].
Bioprocess Performance Targets
For therapeutic compounds like Hydroxysafflor Yellow A (HSYA), achieving commercially viable production levels remains a primary challenge following pathway elucidation. While specific titer data for HSYA in industrial fermentation remains limited in public literature, the research focus has centered on establishing complete biosynthetic pathways as the foundation for future optimization [23]. For many pharmaceutical compounds produced biosynthetically, competitive titers typically exceed 1-5 g/L in established processes, with yields greater than 20% of theoretical maximum and productivities surpassing 0.1 g/L/h representing important milestones toward commercial viability.
Table 2: Industrial Bioprocess Performance Ranges for Pharmaceutical Compounds
| Performance Tier | Titer (g/L) | Yield (g/g) | Productivity (g/L/h) |
|---|---|---|---|
| Early Research | < 0.1 | < 0.05 | < 0.01 |
| Process Development | 0.1 - 1 | 0.05 - 0.15 | 0.01 - 0.05 |
| Pilot Scale | 1 - 5 | 0.15 - 0.25 | 0.05 - 0.15 |
| Commercial Production | > 5 | > 0.25 | > 0.15 |
Manufacturing sectors beyond pharmaceuticals often achieve significantly higher metrics. For instance, benchmark data from 1,500 manufacturing plants demonstrated that productivity increases enabled producing 5 days of product in just 4 days—a 25% efficiency gain that highlights the potential for optimization in bioprocess operations [79].
Experimental Protocols for Metric Determination
Accurate quantification of titer, yield, and productivity requires standardized analytical methodologies and rigorous experimental design. The following protocols outline established procedures for measuring these metrics in biosynthetic production systems.
Analytical Methods for Titer Quantification
High-performance liquid chromatography (HPLC) coupled with various detection systems serves as the gold standard for quantifying target compound concentrations in complex biological matrices.
Sample Preparation: Culture broth should be centrifuged at 10,000 × g for 10 minutes to separate biomass from supernatant. For intracellular compounds, resuspend cell pellet in appropriate extraction solvent (e.g., methanol, acetonitrile/water mixture) and disrupt cells via sonication or bead beating. Following a second centrifugation, filter the supernatant through a 0.22 μm membrane prior to HPLC analysis [23].
HPLC Analysis: Utilize a reverse-phase C18 column (250 × 4.6 mm, 5 μm particle size) maintained at constant temperature (typically 25-40°C). Employ gradient elution with mobile phases consisting of water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). For HSYA quantification, a validated method uses a gradient from 5% to 30% B over 25 minutes with a flow rate of 1.0 mL/min and detection at 275-400 nm [23]. Quantify concentration against a standard curve prepared with authentic reference standards.
Alternative Methods: For compounds lacking chromophores, HPLC coupled to evaporative light scattering detection (ELSD) or refractive index detection (RID) may be employed. Mass spectrometry (LC-MS) provides superior specificity for complex mixtures and enables structural confirmation through fragmentation patterns [23].
Yield Determination Protocols
Calculating yield requires precise measurement of both product formation and substrate consumption throughout the bioprocess timeline.
Substrate Quantification: Track primary carbon source (e.g., glucose, glycerol) concentration using commercial enzymatic assay kits or HPLC with refractive index detection. Collect samples at multiple time points (0, 12, 24, 48 hours, etc.) to monitor substrate depletion kinetics [23].
Yield Calculations: Determine gravimetric yield (Yₚ/ₛ) as grams of product formed per gram of substrate consumed. Calculate molar yield (mol product/mol substrate) for stoichiometric comparisons. For pathway-specific yields, consider the theoretical maximum based on biochemical pathway stoichiometry to express yield as a percentage of theoretical maximum [23].
Productivity Assessment Methods
Productivity calculations integrate both titer and temporal components to measure production rates.
Time-Course Analysis: Conduct experiments where product titer and biomass concentration are measured at regular intervals throughout the cultivation period. For batch processes, total process time includes lag phase, production phase, and any downtime between batches [23].
Productivity Calculations: Determine volumetric productivity as final titer (g/L) divided by total process time (hours). For more nuanced analysis, calculate specific productivity during the exponential production phase by plotting product accumulation versus time and determining the slope of the linear region. Biomass-specific productivity can be calculated by normalizing against cell dry weight (g product/g DCW/h) [23].
Pathway Elucidation Workflow and Metric Integration
The discovery and optimization of biosynthetic pathways represent a multidisciplinary endeavor that integrates increasingly abundant big data with advanced experimental validation. The workflow below illustrates how modern pathway elucidation systematically progresses from gene discovery to the metric-driven evaluation critical for industrial application.
This integrated approach demonstrates how pathway discovery naturally progresses toward the quantification of industrial performance metrics. The initial multi-omics phase generates the comprehensive datasets needed for candidate gene identification [21]. Subsequent functional characterization validates enzyme activities and pathway completeness [23], while the final stage focuses on quantifying the titer, yield, and productivity that determine commercial viability.
Research Reagent Solutions for Biosynthetic Studies
The experimental workflow for pathway elucidation and metric evaluation relies on specialized reagents and biological tools. The following table details essential research solutions and their specific applications in biosynthetic studies.
Table 3: Essential Research Reagents for Biosynthetic Pathway Elucidation
| Reagent / Solution | Function & Application | Specific Examples |
|---|---|---|
| Heterologous Expression Systems | Host organisms for expressing candidate biosynthetic genes and reconstituting pathways | Escherichia coli (prokaryotic), Saccharomyces cerevisiae (yeast), Nicotiana benthamiana (plant) [21] [23] |
| Virus-Induced Gene Silencing (VIGS) Tools | In planta functional validation through targeted gene knockdown | VIGS vectors (e.g., TRV-based) for safflower (Carthamus tinctorius) to confirm gene function in HSYA biosynthesis [23] |
| Enzyme Assay Components | In vitro biochemical characterization of catalytic activity | Purified enzymes, NADPH cofactor, UDP-glucose sugar donor, 2-oxoglutarate for 2OGD enzymes [23] |
| Analytical Standards | Quantification and structural confirmation of metabolites | Authentic reference compounds (e.g., HSYA standard for HPLC calibration) [23] |
| Multi-omics Profiling Kits | Generation of genomics, transcriptomics, and metabolomics datasets | RNA extraction kits, cDNA synthesis kits, next-generation sequencing library prep kits [21] |
These research reagents enable the transition from computational predictions to experimental validation and ultimately to the quantitative assessment of pathway performance. The selection of appropriate expression systems is particularly critical, with each platform offering distinct advantages: microbial systems for rapid screening and plant systems for handling complex eukaryotic enzymes and pathways [21] [23].
The journey from biosynthetic pathway discovery to commercially viable manufacturing is guided by the rigorous application and optimization of titer, yield, and productivity metrics. These quantitative parameters provide the critical link between the groundbreaking research that elucidates complex metabolic pathways—often through innovative integration of big data and multi-omics technologies [21]—and the industrial imperatives of economic feasibility and scalable production. As the recent elucidation of the HSYA pathway demonstrates [23], even the most intricate biosynthetic routes can be deciphered and engineered for enhanced performance. By systematically implementing the experimental protocols, benchmarking standards, and research methodologies outlined in this technical guide, scientists and drug development professionals can effectively translate pathway discoveries into manufacturing successes, ultimately accelerating the delivery of valuable plant-derived therapeutics to patients.
Conclusion
The field of biosynthetic pathway elucidation is undergoing a profound transformation, propelled by integrated multi-omics and artificial intelligence. The convergence of these technologies is systematically dismantling long-standing barriers, enabling the discovery of complex pathways and their efficient engineering in heterologous hosts. Future progress will hinge on the continued development of automated, algorithm-driven platforms and a deeper understanding of cellular processes like aging that impact bioproduction. For biomedical research, these advances promise a more robust and sustainable pipeline for discovering and manufacturing complex therapeutic compounds, ultimately accelerating the journey from natural product discovery to clinical application.
References
- 1. computational methods for biosynthetic pathway design [https://www.sciencedirect.com/science/article/pii/S2405805X25000687]
- 2. Computational biotechnology guides elucidation of the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8254074/]
- 3. Deep learning driven biosynthetic pathways navigation for ... [https://www.nature.com/articles/s41467-022-30970-9]
- 4. Genome mining-driven discovery of enzymes catalyzing ... [https://www.nature.com/articles/s41429-025-00881-0]
- 5. The Paclitaxel Biosynthesis Pathway Unlocked - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC12538117/]
- 6. Ipecac alkaloid biosynthesis in two evolutionarily distant ... [https://www.nature.com/articles/s41589-025-01926-z]
- 7. Two plant species invent the same chemically complex and ... [https://www.ice.mpg.de/495385/PR_Colinas]
- 8. Two Plant Species Independently Produce the Same ... [https://bioengineer.org/two-plant-species-independently-produce-the-same-complex-and-medically-valuable-compound/]
- 9. Ipecac alkaloid biosynthesis in two evolutionarily distant plants [https://pmc.ncbi.nlm.nih.gov/articles/PMC12568652/]
- 10. Is a metabolic enzyme complex involved in the efficient and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3115036/]
- 11. Three New O-Methyltransferases Are Sufficient for All O ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2844217/]
- 12. The New β-D-Glucosidase in Terpenoid-Isoquinoline Alkaloid ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3259899/]
- 13. Back to the Future of Metabolism—Advances in ... [https://www.mdpi.com/2075-1729/14/3/364]
- 14. Discovery of New Enzymatic Functions and Metabolic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8238782/]
- 15. Deep learning gets the glory, deep fact checking gets ignored [https://rachel.fast.ai/posts/2025-06-04-enzyme-ml-fails/]
- 16. computational methods for biosynthetic pathway design [https://pmc.ncbi.nlm.nih.gov/articles/PMC12159830/]
- 17. Systems and synthetic biology for plant natural product ... [https://www.sciencedirect.com/science/article/pii/S2211124725004863]
- 18. Enzyme Miniaturization: Revolutionizing Future Biocatalysts [https://pmc.ncbi.nlm.nih.gov/articles/PMC12226312/]
- 19. A structure-oriented kinetics dataset of enzyme-substrate ... [https://www.nature.com/articles/s41597-025-05829-5]
- 20. Evolutionary Studies Drive the Elucidation of Plant Natural ... [https://communities.springernature.com/posts/evolutionary-studies-drive-the-elucidation-of-plant-natural-product-biosynthetic-pathways]
- 21. From data to discovery: leveraging big data in plant natural ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12183336/]
- 22. Chemoproteomics approach to elucidating biosynthetic ... [https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2024.1506569/full]
- 23. Elucidation of the biosynthetic pathway of hydroxysafflor ... [https://www.nature.com/articles/s41467-025-59774-3]
- 24. MEANtools integrates multi-omics data to identify metabolites ... [https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3003307]
- 25. MEANtools integrates multi-omics data to identify ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12327601/]
- 26. Mass-spectrometry based metabolomics - PubMed Central - NIH [https://pmc.ncbi.nlm.nih.gov/articles/PMC12105183/]
- 27. Opportunities and challenges in the application of ... [https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2025.1684057/full]
- 28. MetaboAnalyst [https://www.metaboanalyst.ca/]
- 29. The 2025 Bioinformatics Toolkit: From Sample Prep to AI- ... [https://blog.omni-inc.com/blog/the-2025-bioinformatics-toolkit-from-sample-prep-to-ai-driven-analysis]
- 30. Global trends in machine learning applications for single-cell ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12357469/]
- 31. Identifying differentially expressed genes (DEGs) ... [https://www.novogene.com/us-en/resources/blog/how-to-employ-statistical-approaches-to-identify-differentially-expressed-genes-deg/]
- 32. Differential gene expression analysis pipelines and ... [https://www.sciencedirect.com/science/article/pii/S2001037024000424]
- 33. A Metabolomics and Transcriptomics Resource for Identifying ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12508522/]
- 34. Dynamic identification of candidate genes associated with ... [https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2025.1613360/full]
- 35. Transcriptome analysis reveals candidate genes involved ... [https://www.nature.com/articles/s41598-025-00794-w]
- 36. AI in Pharma and Biotech: Market Trends 2025 and Beyond [https://www.coherentsolutions.com/insights/artificial-intelligence-in-pharmaceuticals-and-biotechnology-current-trends-and-innovations]
- 37. From Lab to Clinic: How Artificial Intelligence (AI) Is ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12298131/]
- 38. Predicting Biological Activity from Biosynthetic Gene ... [https://pubmed.ncbi.nlm.nih.gov/40524326/]
- 39. AI Predictive Analytics in 2025: Trends, Tools, and ... [https://superagi.com/ai-predictive-analytics-in-2025-trends-tools-and-techniques-for-forward-thinking-businesses/]
- 40. Predictive AI Use Cases, Architecture, and Business Value [https://aerospike.com/blog/predictive-ai/]
- 41. How AI will reshape pharma in 2025 [https://www.drugtargetreview.com/article/154981/how-ai-will-reshape-pharma-by-2025/]
- 42. Plant synthetic biology: from knowledge to biomolecules [https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2025.1562216/full]
- 43. Plant synthetic biology: from knowledge to biomolecules - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC12602438/]
- 44. Metabolic engineering in Nicotiana benthamiana [https://link.springer.com/article/10.1007/s42994-025-00234-3]
- 45. Decoding Natural Product Biosynthesis: Next-Generation ... [https://panomebio.com/blog/decoding-natural-product-biosynthesis-next-generation-metabolomics-approaches-for-pathway-elucidation/]
- 46. Reducing biomass recalcitrance by heterologous ... [https://www.nature.com/articles/s41598-017-16909-x]
- 47. Relieving metabolic burden to improve robustness and ... [https://www.sciencedirect.com/science/article/pii/S0734975024000958]
- 48. Hierarchical metabolic engineering for rewiring cellular ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12514951/]
- 49. RAIChU: automating the visualisation of natural product ... [https://jcheminf.biomedcentral.com/articles/10.1186/s13321-024-00898-x]
- 50. Spatially organizing biochemistry: choosing a strategy to ... [https://www.nature.com/articles/s41598-018-26399-0]
- 51. Compartment engineering in yeast: a path to increased ... [https://www.sciencedirect.com/science/article/abs/pii/S1385894725101228]
- 52. Compartmentalization and transporter engineering strategies ... [https://microbialcellfactories.biomedcentral.com/articles/10.1186/s12934-022-01819-z]
- 53. Unlocking the potential of flavonoid biosynthesis through ... [https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2025.1597007/full]
- 54. The connection between aging, cellular senescence and gut ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11464129/]
- 55. PathVisio biological pathway editor | pathvisio.github.io [https://pathvisio.org/]
- 56. Molecular insights fast-tracked: AI in biosynthetic pathway ... [https://pubs.rsc.org/en/content/articlelanding/2025/np/d4np00003j]
- 57. An industrial automated laboratory for programmable ... [https://www.nature.com/articles/s44286-025-00303-w]
- 58. Automated strain construction for biosynthetic pathway ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12611344/]
- 59. Automating microbial strain development for a sustainable ... [https://www.tecan.com/tecan-journal/automating-microbial-strain-development-for-a-sustainable-future]
- 60. Accelerating enzyme discovery and engineering with high ... [https://pubs.rsc.org/en/content/articlehtml/2025/np/d4np00031e]
- 61. Guide to Enzyme Unit Definitions and Assay Design [https://resources.biomol.com/biomol-blog/guide-to-enzyme-unit-definitions-and-assay-design]
- 62. Validating Identity, Mass Purity and Enzymatic Purity of ... - NCBI [https://www.ncbi.nlm.nih.gov/books/NBK91995/]
- 63. In vitro deamination assay to measure the activity and ... [https://pubmed.ncbi.nlm.nih.gov/40250961/]
- 64. Glycosyltransferase enzymatic assays: Overview and ... [https://www.sciencedirect.com/science/article/abs/pii/S0003269725000648]
- 65. A live bacteria enzyme assay for identification of human ... [https://www.nature.com/articles/s41551-025-01391-y]
- 66. Using Color-Coding for Organized Sample Tracking [https://computype.com/blog/color-coding-for-organized-sample-tracking/?srsltid=AfmBOor5uLzpg0JWzGSEYiCKQ0kqGbyVCO76D1wB_hAYMQqx72gOiyyd]
- 67. Standard Color Codes for Specimen Vials [https://www.needle.tube/resources-26/Standard-Color-Codes-for-Specimen-Vials:-A-Guide-for-Healthcare-Professionals]
- 68. Metabolomics: Insights for Disease Mechanisms & ... [https://esmed.org/metabolomics-insights-for-disease-mechanisms-biomarkers/]
- 69. Recent Advances in Liquid Chromatography–Mass ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12204688/]
- 70. NMR-based metabolomics: Where are we now and where ... [https://www.sciencedirect.com/science/article/abs/pii/S0079656525000081]
- 71. Comparison analysis of metabolite profiling in seeds and bark ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9629078/]
- 72. Metabolomics reveals differences in metabolite profiles ... [https://www.sciencedirect.com/science/article/pii/S1878535223008924]
- 73. Identification and Profiling of Primary Metabolites Through ... [https://pubmed.ncbi.nlm.nih.gov/39885026/]
- 74. Navigating the challenges of engineering composite ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11910955/]
- 75. Designing pathways for bioproducing complex chemicals ... [https://www.nature.com/articles/s41467-025-59827-7]
- 76. Modeling host–pathway dynamics at the genome scale ... [https://www.sciencedirect.com/science/article/pii/S1096717625000849]
- 77. Ten simple rules for creating reusable pathway models for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8375987/]
- 78. Productivity Benchmarks Report 2025: Cross-Industry & ... [https://www.activtrak.com/blog/productivity-benchmarks/]
- 79. 2025 Productivity Benchmark Report - Redzone [https://rzsoftware.com/report/productivity-benchmark-report/]